Navegação por Autores IPEN "BARBEZAN, ANGELICA B."
- Página inicial
- →
- Navegação por Autores IPEN
- Sobre
- Perfil Técnico
- Política de funcionamento
- Ajuda
- Apresentação
Navegação por Autores IPEN "BARBEZAN, ANGELICA B."
Itens para a visualização no momento 1-12 de 12
-
. Ames test to detect mutagenicity of 2-alkylcyclobutanones: a review. Journal of Food Science, v. 00, n. 0, p. 1-5, 2017. DOI: 10.1111/1750-3841.13721 Abstract: Food irradiation is an effective and safe method for preservation and long-term storage, and it is approved for use in over 60 countries for various applications in a wide variety of food products. This process is performed by use of accelerated electron beams, X-rays, or gamma radiation (60Co or 137Cs). 2-Alkylcyclobutanones (2-ACBs) are the only known radiolytic products generated from foods that have fatty acids (triglycerides) and are subjected to irradiation. Since the 1990s toxicological safety studies of 2-ACBs have been conducted extensively through synthetic compounds, then and tests to determine if the compounds have any mutagenic activity are strictly necessary. The Ames test was chosen by many researchers to assess the mutagenicity of 2-ACBs. The test uses distinct bacterial cell lines Salmonella typhimurium to detect point mutations at sites guanine–cytosine (G–C) and Escherichia coli to detect point mutations at sites adenine–thymine (A–T). This bibliographic research aims to bring together all the results obtained and a comparison and cell lines used, type of plates, and solvents. This research showed that no mutagenic activity was observed in any of the cell lines and concentrations evaluated by the works of authors, so the 2-ACBs compounds showed no mutagenic substance in concentrations detectable by the Ames test.
Palavras-Chave: food processing; irradiation; mutagenesis; mutations; butanols; cycloalkanes; testing; americium
. Ames test to detect mutagenicity of 2-alkylcyclobutanones: a review. Journal of Food Science, v. 00, n. 0, p. 1-5, 2017. DOI: 10.1111/1750-3841.13721. Disponível em: http://repositorio.ipen.br/handle/123456789/27595. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Anti-HER2 monoclonal antibody based-radioimmunoconjugates: assessment of the chelating agent influence. Bioorganic & Medicinal Chemistry, v. 33, p. 1-9, 2021. DOI: 10.1016/j.bmc.2021.115996 Abstract: In the present work, the radioimmunoconjugates 111In-DTPA-trastuzumab and 177Lu-DOTA-trastuzumab were evaluated regarding the influence of the chelating agents on the physical–chemical parameters and human epidermal growth factor receptor 2 (HER2) tumor cell binding. Data showed that both chelating agents, at predetermined molar ratios (antibody:chelator 1:10 and 1:20), did not influence the immunoconjugates integrity, the radiolabeling process and the radiolabeled antibodies stability. However, differences were observed in the lipophilic feature between DOTA and DTPA radioimmunoconjugates and in the specific binding to SK-BR-3 tumor cells (HER2 positive). Therefore, this study showed the importance of assessing the influence of chelating agents and their molar ratios in the development process of radioimmunoconjugates.
Palavras-Chave: tumor cells; neoplasms; drugs; therapeutic uses; radioimmunotherapy; chelating agents; radioisotopes
. Anti-HER2 monoclonal antibody based-radioimmunoconjugates: assessment of the chelating agent influence. Bioorganic & Medicinal Chemistry, v. 33, p. 1-9, 2021. DOI: 10.1016/j.bmc.2021.115996. Disponível em: http://repositorio.ipen.br/handle/123456789/31971. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Comparação da marcação de diversos fosfonatos: MDP, EDTMP e clodronato com sup(188)Re / Comparison of labeling various phosphonates: MDP, EDTMP and clodronate with sup(188)Re . 2012. Dissertação (Mestrado) - Instituto de Pesquisas Energeticas e Nucleares - IPEN-CNEN/SP, São Paulo. 94 p. Orientador: João Alberto Osso Junior. DOI: 10.11606/D.85.2012.tde-16012013-091715 Abstract: A grande aplicação dos radiofármacos está em medicina nuclear diagnóstica representando 95% dos procedimentos realizados, porém, nos últimos anos, tem crescido consideravelmente a sua aplicação em procedimentos terapêuticos. Os radionuclídeos que emitem particulas ionizantes (α, β e elétrons Auger) são indicados para tratamento de tumores. Tumores malignos são responsáveis por aproximadamente 12% dos óbitos e representam a terceira causa de mortalidade no Brasil. O 188Re é um dos mais atrativos radioisótopos para uma variedade de aplicações terapêuticas em medicina nuclear, oncologia e intervenções cardiológicas, é totalmente favorável e conveniente pelo fato de que ele é livre de carregador e pode ser obtido de forma econômica na forma de um gerador de 188W/188Re, além de possuir uma meia-vida fisica de 16,9 horas e 100% de emissão de radiação β-. A partir da década de 2000 vêm sendo realizadas diversas investigações envolvendo marcações de moléculas com 188Re. Os tumores metastáticos são a forma mais comum de malignidade esquelética. Em casos metastáticos os principais objetivos do tratamento são a prevenção de fraturas patológicas e promover a sobrevida com o máximo de preservação de função permitindo que o paciente mantenha o máximo possível de mobilidade e controle da dor. O objetivo deste trabalho foi realizar a comparação das marcações de diversos fosfonatos (Metileno disfofonato de Sódio MDP, Ácido Etilenodiaminotetrametilenofosfônico EDTMP, e do diclorometilenobifosfonato de sódio - Clodronato) com 188Re para terapia de metastáses ósseas. Fosfonatos são inibidores da reabsorção óssea osteoclástica e são efetivos neste tratamento. As marcações do MDP, EDTMP e Clodronato com 188Re foram otimizadas utilizando como agente redutor o cloreto estanoso (SnCl2 2H2O) e como agente estabilizante o ácido ascórbico. As variáveis estudadas foram massa do ligante, massa do SnCl2.2H2O, massa do ácido ascórbico, tempo, pH e temperatura da reação. Os resultados mostraram que se obteve um excelente rendimento de marcação de 98% para o 188Re-MDP, de 83% para o 188Re-EDTMP e 85% para o 188Re-Clodronato.
Palavras-Chave: radiotherapy; radiopharmaceuticals; drugs; labelled compounds; comparative evaluations; phosphonates; organic phosphorus compounds; tungsten; rhenium; neoplasms; cardiovascular diseases; tumor cells
. Comparação da marcação de diversos fosfonatos: MDP, EDTMP e clodronato com sup(188)Re. Orientador: João Alberto Osso Junior. 2012. 94 f. Dissertação (Mestrado) - Instituto de Pesquisas Energeticas e Nucleares - IPEN-CNEN/SP, São Paulo. DOI: 10.11606/D.85.2012.tde-16012013-091715. Disponível em: http://repositorio.ipen.br/handle/123456789/10150. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Development of radioactive nanoparticles functionalized with gum arabic to be used in nanobrachytherapy. In: BRAZIL MRS MEETING, 21st, October 1-5, 2023, Maceió, AL. Abstract... São Carlos, SP: Aptor Software, 2023. p. 553-553. Abstract: The development of new materials emerges as an alternative to the treatment of cancer, from the combination of nanotechnology and brachytherapy a new area of research was born, Nanobrachytherapy, which through the properties of nanometric materials can achieve better results in the fight against cancer. The objective of this work is classified as radiotherapy, which consists of the use of ionizing radiation to destroy or inhibit the growth of abnormal cells that form a tumor. [1] The ability to integrate NPsAu into biological systems is due to the nanometric dimensions of NPsAu probes which facilitate their incorporation into biological systems, as well as their bioconjugation and non-cytotoxic potential. [2] Taking into account the previous objective, gold was selected as the base element for obtaining nanometric systems, which due to its chemical richness and especially due to the intrinsic properties of one of its radioisotopes, which would allow us in theory to meet the stated objective. . Initially, it was based on the knowledge of the literature, and non-radioactive nanometric systems were obtained, and after a series of stability, characterization and application tests, the radioactive nanometric systems were obtained. Working with radioactive systems posed a great challenge, and up to now it presents us with situations to solve, but we have managed to create a methodology for obtaining, characterizing and applying radioactive gold nanoparticles, and also obtaining positive results from their application.. Development of radioactive nanoparticles functionalized with gum arabic to be used in nanobrachytherapy. In: BRAZIL MRS MEETING, 21st, October 1-5, 2023, Maceió, AL. Abstract... São Carlos, SP: Aptor Software, 2023. p. 553-553. Disponível em: http://repositorio.ipen.br/handle/123456789/34559. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Flow cytometry based micronucleus assay for evaluation of genotoxic potential of 2-ACBs in hepatic cells HepG2. Brazilian Journal of Radiation Sciences, v. 7, n. 2A, p. 1-24, 2019. DOI: 10.15392/bjrs.v7i2A.684 Abstract: Food irradiation is approved for use in more than 60 countries for applications and purposes in a wide variety of foods, being an effective and safe method for preservation and long-term storage. 2- Alkylcyclobutanones (2-ACBs) are the only known radiolytic products generated from foods that contain fatty acids (Triglycerides) when irradiated. The acids analyzed in this study are palmitic and stearic, which when irradiated form 2-Dodecylcyclobutanones (2-dDCB) and 2-Tetradecylcyclobutanone (2-tDCB). Part of the 2-ACBs ingested is excreted through feces and part is deposited in adipose tissues. In vitro studies so far have been only in colon cells. The work used a human hepatoma cell line (HepG2) since the accumulation of fat in this organ is quite common. Micronucleus test was selected to evaluate possible genotoxic effects of 2-dDCB and 2-tDCB compounds when exposed to high concentrations (447, 1422 and 2235 μM) for 4 and 24 hours. Tests were performed in quadriplicates using flow cytometric analysis. None detectable genotoxic damage was observed after 4 hours of exposure to the compounds, and cytotoxic effects were only significant at the highest concentration (2235 μM) of 2-dDCB. After 24 hours of exposure, slight genotoxic damage was observed at all concentrations evaluated, and cytotoxic effects were only present when exposed to compound 2-tDCB. Although there is a genotoxic and cytotoxic effect in some of the situations tested, the two compounds predominantly induced proliferation reduction effects of this hepatic tumor cell line.
Palavras-Chave: food processing; irradiation; hexadecanoic acid; cell flow systems; dodecyl radicals; hepatomas; in vitro; octadecanoic acid; radiation effects; toxicity; carcinomas
. Flow cytometry based micronucleus assay for evaluation of genotoxic potential of 2-ACBs in hepatic cells HepG2. Brazilian Journal of Radiation Sciences, v. 7, n. 2A, p. 1-24, 2019. DOI: 10.15392/bjrs.v7i2A.684. Disponível em: http://repositorio.ipen.br/handle/123456789/29987. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Flow cytometry based micronucleus assay for evaluation of genotoxic potential of 2-ACBs in hepatic cells HepG2. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 22-27, 2017, Belo Horizonte, MG. Proceedings... Rio de Janeiro, RJ: Associação Brasileira de Energia Nuclear, 2017. Abstract: Food irradiation is approved for use in more than 60 countries for applications and purposes in a wide variety of foods, being an effective and safe method for preservation and long-term storage. 2-Alkylcyclobutanones (2-ACBs) are the only known radiolytic products generated from foods that contain fatty acids (Triglycerides) when irradiated. The acids analyzed in this study are palmitic and stearic, which when irradiated form 2-Dodecylcyclobutanones (2-dDCB) and 2-Tetradecylcyclobutanone (2-tDCB). Part of the 2-ACBs ingested is excreted through feces and part is deposited in adipose tissues. In vitro studies so far have been only in colon cells. The work used a human hepatoma cell line (HepG2) since the accumulation of fat in this organ is quite common. Micronucleus test was selected to evaluate possible genotoxic effects of 2-dDCB and 2-tDCB compounds when exposed to high concentrations (447, 1422 and 2235 μM) for 4 and 24 hours. Tests were performed in quadriplicates using flow cytometric analysis. None detectable genotoxic damage was observed after 4 hours of exposure to the compounds, and cytotoxic effects were only significant at the highest concentration (2235 μM) of 2-dDCB. After 24 hours of exposure, slight genotoxic damage was observed at all concentrations evaluated, and cytotoxic effects were only present when exposed to compound 2-tDCB. Although there is a genotoxic and cytotoxic effect in some of the situations tested, the two compounds predominantly induced proliferation reduction effects of this hepatic tumor cell line.
Palavras-Chave: cell flow systems; dodecyl radicals; food processing; hepatomas; hexadecanoic acid; in vitro; irradiation; octadecanoic acid; radiation effects; toxicity
. Flow cytometry based micronucleus assay for evaluation of genotoxic potential of 2-ACBs in hepatic cells HepG2. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 22-27, 2017, Belo Horizonte, MG. Proceedings... Rio de Janeiro, RJ: Associação Brasileira de Energia Nuclear, 2017. Disponível em: http://repositorio.ipen.br/handle/123456789/28220. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Gold nanoparticles stabilized with gum arabic for cancer treatment. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 21-25, 2019, Santos, SP. Proceedings... Rio de Janeiro: Associação Brasileira de Energia Nuclear, 2019. p. 1946-1952. Abstract: Cancer is a group of diseases characterized by uncontrolled growth and abnormal spread of cells. The number of deaths due to cancer is higher than the ones caused by AIDS, tuberculosis and malaria combined. Among the different options of cancer treatment radiotherapy (teletherapy and brachytherapy) sta nds out. The presence of gold nanoparticles (AuNPs) may enhance energy deposition for teletherapy treatment. The use of nanoparticles for brachytherapy have been studied, and AuNPs is a good option once they can easily permeate tumor vasculature and remain in tumors. However, the tumor uptake of AuNPs may be significantly reduced due the attenuation with the formation of the protein coronas. The objective of this work is present the functionalization of AuNPs with arabic gum (GA). GA is a biocompatible, non toxic, water soluble, natural gum obtained from Acacia senegal tree. In this study, a synthesis of gold nanoparticles (AuNPs), based on Turkevich method, using citrate (NaCit) solution as reducing agent and a HAuCl4 solution, under vigorously stirring and boiling temperature, going from a light yellow to a wine red in three minutes. The functionalization of the nanoparticles was performed with Arabic gum solutions, in three different concentrations , which were a dded under stirring to the AuNPs already obtai ned. Samples were characterized to measure the size of the samples. Lower concentrations o GA in the solution presented smaller coated particles (up to 45 nm).
Palavras-Chave: brachytherapy; gold; gums; nanoparticles; neoplasms; radiotherapy; therapy
. Gold nanoparticles stabilized with gum arabic for cancer treatment. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 21-25, 2019, Santos, SP. Proceedings... Rio de Janeiro: Associação Brasileira de Energia Nuclear, 2019. p. 1946-1952. Disponível em: http://repositorio.ipen.br/handle/123456789/30592. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. In vivo genotoxicity of 2-Alkylcyclobutanones in liver cells from rats fed with irradiated cocoa butter using flow citometry. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 21-25, 2019, Santos, SP. Proceedings... Rio de Janeiro: Associação Brasileira de Energia Nuclear, 2019. p. 2070-2078. Abstract: Food irradiation proves to be an effective technique of eliminating some pathogens from food and this has gained significant attention to its potential for food safety. Since 1990, studies on the toxicological safety of 2-Alkylcyclobutanones have been conducted extensively. 2- Alkylcyclobutanones are unique radiolytic products generated by the radiation-induced breakage of triglycerides in food, are exclusively found in irradiated lipid containing foods. 2-dodecylcyclobutanone (2-dDCB) and 2-tetradecylcyclobutanone (2-tDCB) are the predominant compounds detected in irradiated food. Despite studies showing non- genotoxicity of 2-ACBs (2-Alkylcyclobutanones), the results are conflicting and therefore we continue the studies in order to confirm the compounds safety for human health. In vivo micronucleus test were performed to verify the 2-ACBs genotoxic effects in hepatic cells using flow citometry. We used cocoa butter irradiated with 20 kGy at IPEN GAMACELL. A group with animals (IPEN Ethical Animal Experimentation Committee, process number 148/14) was treated with daily intake of irradiated cocoa butter, synthesized 2- Dodecylcyclobutanone and 2-Tetradecylcyclobutanone for two months. Hepatic cells were selected for genotoxicity analysis due to the liver importance in the compounds metabolization. Analyzes were made by micronucleus test with specific cells extracted from hepatic tissue using flow cytometry, which is an alternative to conventional techniques, allowing faster analysis and reduction in the animals number that is a subject much approached in research today. The improvement of the analytical techniques is important for the research future since the irradiation process is already consolidated. The results confirmed the safety of the food irradiation process, as they did not indicate the genotoxic potential of the samples.
Palavras-Chave: alkyl radicals; butter; cell flow systems; cocoa products; food processing; genetic effects; liver cells; mice; radiation doses; toxicity
. In vivo genotoxicity of 2-Alkylcyclobutanones in liver cells from rats fed with irradiated cocoa butter using flow citometry. In: INTERNATIONAL NUCLEAR ATLANTIC CONFERENCE, October 21-25, 2019, Santos, SP. Proceedings... Rio de Janeiro: Associação Brasileira de Energia Nuclear, 2019. p. 2070-2078. Disponível em: http://repositorio.ipen.br/handle/123456789/30603. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Potential evaluation of mutagenic compounds 2-dodecylcyclobutanone and 2-tetradecylcyclobutanone through the Ames test. In: INTERNATIONAL MEETING ON RADIATION PROCESSING, 18th, November 07-11, 2016, Vancouver, BC, Canada. Abstract... 2016. Abstract: Food irradiation is an effective and safe method for preservation and long-term storage for various applications in a wide variety of food products. This process is performed by the use of accelerated electron beams, X-rays or gamma radiation. The 2-Alkylcyclobutanones (2-ACBs) are the only known radiolytic products generated in foods that have fatty acids and were subjected to irradiation. In this study, the stearic and palmitic acid was analyzed when irradiated medium 2-Tetradecylcyclobutanone (2-tDCB) and 2-Dodecylcyclobutanone (2-dDCB). Since the 1990s toxicological safety studies of 2-ACBs have been conducted extensively through synthetic compounds. Additionally, tests to determine if the compounds have any mutagenic activity are strictly necessary. The Ames test was the test choosen for assessing the genotoxicity of both 2- dDCB and 2tDCB compounds. It was used five different bacterial strains TA-1535, TA-1537, TA 98 and TA 100 Salmonella typhimurium, to detect mutations in specific sites Guanine-Cytosine (GC) and WP2 uvrA, Escherichia coli was used to detect point mutations at sites of Adenine- Thymine (AT). This research, unlike those undertaken by other authors up to now, presents some particularities in their factors for a more complete assessment, such as the use of all necessary cell lines to identify possible mutations in specific sites, changes in solvent commonly used, appropriate plates and the investigation of possible mutagenic effects on two important compounds 2-dDCB and 2-tDCB simultaneously. The study revealed no mutagenic activity in any of the cell lines and concentrations evaluated. In conclusion, the compounds 2-dDCB and 2tDCB showed no mutagenic effect in concentrations detectable by the AMES test.. Potential evaluation of mutagenic compounds 2-dodecylcyclobutanone and 2-tetradecylcyclobutanone through the Ames test. In: INTERNATIONAL MEETING ON RADIATION PROCESSING, 18th, November 07-11, 2016, Vancouver, BC, Canada. Abstract... 2016. Disponível em: http://repositorio.ipen.br/handle/123456789/27461. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Radiolabeled GX1 peptide for tumor angiogenesis imaging. Applied Biochemistry and Biotechnology, p. 1-12, 2018. DOI: 10.1007/s12010-018-2700-z Abstract: Early and accurate detection of primary or metastatic tumors is of great value in staging, treatment management, and prognosis. Tumor angiogenesis plays an essential role in the growth, invasion, and metastatic spread of solid cancers, and so, is a promising approach for tumor imaging. The GX1 (CGNSNPKSC) peptide was identified by phage display library and has been investigated as a marker for human cancers. This study aims to evaluate the 99mTc-HYNIC-PEG4-c (GX1) as a biomarker for tumor imaging. Our results showed that GX1 specifically binds to tumor cells in vitro. SKMEL28 and MDA-MB231 cells achieved total binding peak at 60 min of incubation. For B16F10 and MKN45 cells, the total and specific binding were similar during all time points, while A549 cell line showed rapid cellular total uptake of the tracer at 30 min of incubation. Biodistribution showed low non-specific uptakes and rapid renal excretion. Melanoma tumors showed enhanced GX1 uptake in animal model at 60 min, and it was significantly blocked by cold peptide. The radiotracer showed tumor specificity, especially in melanomas that are highly vascularized tumors. In this sense, it should be considered in future studies, aiming to evaluate degree of angiogenesis, progression, and invasion of tumors.
Palavras-Chave: bacteriophages; biological markers; melanomas; metastases; radiopharmaceuticals; technetium 99; tracer techniques; tumor cells
. Radiolabeled GX1 peptide for tumor angiogenesis imaging. Applied Biochemistry and Biotechnology, p. 1-12, 2018. DOI: 10.1007/s12010-018-2700-z. Disponível em: http://repositorio.ipen.br/handle/123456789/28981. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Synthesis, activation and application testing of gold nanoparticles for nanobrachytherapy. In: INTERNATIONAL SYMPOSIUM ON TRENDS IN RADIOPHARMACEUTICALS, April 17-21, 2023, Vienna, Austria. Abstract... Vienna, Austria: International Atomic Energy Agency - IAEA, 2023. p. 24-24. Abstract: For more than 50 years, the Energy and Nuclear Research Institute IPEN, has been offering solutions to Brazil through nuclear technology. Thus, one of the main areas where IPEN has contributed assertively is medicine. Reaching the level of 32 radiopharmaceuticals and radioactive sources intended both for therapy and for the diagnosis of several pathologies, including cancer, which are obtained with the help of the two nuclear reactors and two cyclotrons present in the institution. The Institute has a team for the development, production and distribution of radioactive sources for brachytherapy, such as 192 Ir wires and 125 I seeds. Brachytherapy is a cancer treatment technique where the radioactive source is placed close to or in contact with the lesion. The great advantage of the technique is to save healthy tissues. Currently, we are working on obtaining nanometric materials that can be applied in the emerging nano brachytherapy, because of its properties and characteristics at the nanometric level, gold has been the subject of studies and tests. Elemental Au gold can be activated 198 Au inside a nuclear reactor, and has β- decay and a half-life of 2.7 days, which makes it ideal for short-term irradiations. In addition, gold in the form of nanoparticles has a completely different chemistry, with gold nanoparticles (AuNPs) being easily functionalized by a large part of molecular and polymeric binders, which may present favorable characteristics for the studies, and together with AuNPs they are able to work synergistically to achieve greater efficiencies. Currently, AuNPs have been successfully functionalized with gum arabic (GA), a coating widely used in the cosmetic and food industry, which is low cost and along with nanoparticles has shown biocompatibility with different cell groups and has been shown to be very stable over time. The project includes studies regarding the synthesis of nanoparticles, coating, cytotoxicity of AuNPs in vitro "cold" (non-radioactive) and the development of activation protocols in the nuclear reactor. In the next phase, after activation, in the reactor, "hot" tests will be performed in vitro and in vivo.. Synthesis, activation and application testing of gold nanoparticles for nanobrachytherapy. In: INTERNATIONAL SYMPOSIUM ON TRENDS IN RADIOPHARMACEUTICALS, April 17-21, 2023, Vienna, Austria. Abstract... Vienna, Austria: International Atomic Energy Agency - IAEA, 2023. p. 24-24. Disponível em: http://repositorio.ipen.br/handle/123456789/34543. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Synthesis, in vitro testing, and biodistribution of surfactant-free radioactive nanoparticles for cancer treatment. Nanomaterials, v. 12, n. 2, p. 1-13, 2022. DOI: 10.3390/nano12020187 Abstract: New forms of cancer treatment, which are effective, have simple manufacturing processes, and easily transportable, are of the utmost necessity. In this work, a methodology for the synthesis of radioactive Gold-198 nanoparticles without the use of surfactants was described. The nuclear activated Gold-198 foils were transformed into H198AuCl4 by dissolution using aqua regia, following a set of steps in a specially designed leak-tight setup. Gold-198 nanoparticles were synthesized using a citrate reduction stabilized with PEG. In addition, TEM results for the non-radioactive product presented an average size of 11.0 nm. The DLS and results for the radioactive 198AuNPs presented an average size of 8.7 nm. Moreover, the DLS results for the PEG-198AuNPs presented a 32.6 nm average size. Cell line tests showed no cytotoxic effect in any period and the concentrations were evaluated. Furthermore, in vivo testing showed a high biological uptake in the tumor and a cancer growth arrest.
Palavras-Chave: radioactivity; nanoparticles; brachytherapy; neoplasms; testing; in vivo; in vitro; gold 198; distribution
. Synthesis, in vitro testing, and biodistribution of surfactant-free radioactive nanoparticles for cancer treatment. Nanomaterials, v. 12, n. 2, p. 1-13, 2022. DOI: 10.3390/nano12020187. Disponível em: http://repositorio.ipen.br/handle/123456789/33035. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
Itens para a visualização no momento 1-12 de 12
Buscar no repositório
Navegar
Minha conta
Visualizar
A pesquisa no RD utiliza os recursos de busca da maioria das bases de dados. No entanto algumas dicas podem auxiliar para obter um resultado mais pertinente.
✔ É possível efetuar a busca de um autor ou um termo em todo o RD, por meio do Buscar no Repositório , isto é, o termo solicitado será localizado em qualquer campo do RD. No entanto esse tipo de pesquisa não é recomendada a não ser que se deseje um resultado amplo e generalizado.
✔ A pesquisa apresentará melhor resultado selecionando um dos filtros disponíveis em Navegar
✔ Os filtros disponíveis em Navegar tais como: Coleções, Ano de publicação, Títulos, Assuntos, Autores, Revista, Tipo de publicação são autoexplicativos. O filtro, Autores IPEN apresenta uma relação com os autores vinculados ao IPEN; o ID Autor IPEN diz respeito ao número único de identificação de cada autor constante no RD e sob o qual estão agrupados todos os seus trabalhos independente das variáveis do seu nome; Tipo de acesso diz respeito à acessibilidade do documento, isto é , sujeito as leis de direitos autorais, ID RT apresenta a relação dos relatórios técnicos, restritos para consulta das comunidades indicadas.

A opção Busca avançada utiliza os conectores da lógica boleana, é o melhor recurso para combinar chaves de busca e obter documentos relevantes à sua pesquisa, utilize os filtros apresentados na caixa de seleção para refinar o resultado de busca. Pode-se adicionar vários filtros a uma mesma busca.
Exemplo:
Buscar os artigos apresentados em um evento internacional de 2015, sobre loss of coolant, do autor Maprelian.
Autor: Maprelian
Título: loss of coolant
Tipo de publicação: Texto completo de evento
Ano de publicação: 2015
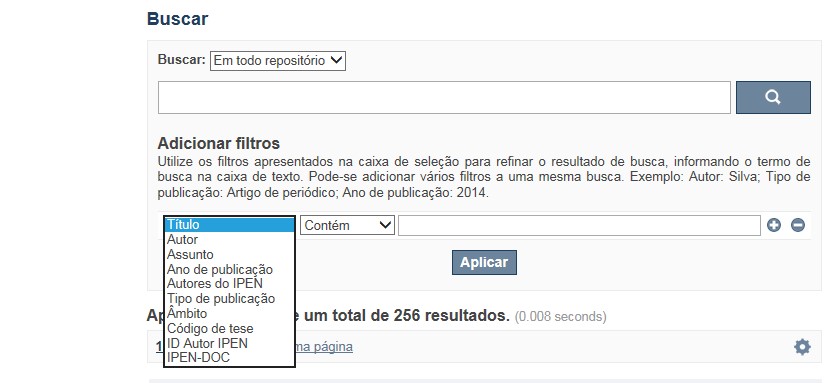
✔ Para indexação dos documentos é utilizado o Thesaurus do INIS, especializado na área nuclear e utilizado em todos os países membros da International Atomic Energy Agency – IAEA , por esse motivo, utilize os termos de busca de assunto em inglês; isto não exclui a busca livre por palavras, apenas o resultado pode não ser tão relevante ou pertinente.
✔ 95% do RD apresenta o texto completo do documento com livre acesso, para aqueles que apresentam o ![]() significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
✔ Ao efetuar a busca por um autor o RD apresentará uma relação de todos os trabalhos depositados no RD. No lado direito da tela são apresentados os coautores com o número de trabalhos produzidos em conjunto bem como os assuntos abordados e os respectivos anos de publicação agrupados.
✔ O RD disponibiliza um quadro estatístico de produtividade, onde é possível visualizar o número dos trabalhos agrupados por tipo de coleção, a medida que estão sendo depositados no RD.
✔ Na página inicial nas referências são sinalizados todos os autores IPEN, ao clicar nesse símbolo ![]() será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
✔ Na página do pesquisador, é possível verificar, as variações do nome, a relação de todos os trabalhos com texto completo bem como um quadro resumo numérico; há links para o Currículo Lattes e o Google Acadêmico ( quando esse for informado).
ATENÇÃO!
ESTE TEXTO "AJUDA" ESTÁ SUJEITO A ATUALIZAÇÕES CONSTANTES, A MEDIDA QUE NOVAS FUNCIONALIDADES E RECURSOS DE BUSCA FOREM SENDO DESENVOLVIDOS PELAS EQUIPES DA BIBLIOTECA E DA INFORMÁTICA.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
1. Portaria IPEN-CNEN/SP nº 387, que estabeleceu os princípios que nortearam a criação do RDI, clique aqui.
2. A experiência do Instituto de Pesquisas Energéticas e Nucleares (IPEN-CNEN/SP) na criação de um Repositório Digital Institucional – RDI, clique aqui.
O Repositório Digital do IPEN é um equipamento institucional de acesso aberto, criado com o objetivo de reunir, preservar, disponibilizar e conferir maior visibilidade à Produção Científica publicada pelo Instituto, desde sua criação em 1956.
Operando, inicialmente como uma base de dados referencial o Repositório foi disponibilizado na atual plataforma, em junho de 2015. No Repositório está disponível o acesso ao conteúdo digital de artigos de periódicos, eventos, nacionais e internacionais, livros, capítulos, dissertações, teses e relatórios técnicos.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
