Navegação IPEN por Autores IPEN "AFFONSO, REGINA"
- Página inicial
- →
- IPEN
- →
- Navegação IPEN por Autores IPEN
- Sobre
- Perfil Técnico
- Política de funcionamento
- Ajuda
- Apresentação
Navegação IPEN por Autores IPEN "AFFONSO, REGINA"
Itens para a visualização no momento 1-20 de 26
-
. Adenocarcinoma prostático: análise clínica e epidemiológica / Prostate adenocarcinoma: clinical and epidemiological analysis. Revista da Sociedade Brasileira de Clínica Médica, v. 15, n. 03, p. 178-182, 2017. Abstract: OBJETIVO: Realizar análise clínica e epidemiológica de pacientes com câncer de próstata. MÉTODOS: Estudo retrospectivo, descritivo de 607 prontuários de pacientes com câncer de próstata, atendidos entre 2012 a 2014. As variáveis analisadas foram: procedência, faixa etária, antígeno prostático específico (PSA) total, escore de Gleason da biópsia e da peça cirúrgica. A análise estatística foi realizada com software SPSS, versão 19.0. RESULTADOS: A maioria dos pacientes (57%) era de Ipatinga (MG) e arredores. A faixa etária mais frequente foi de 61 a 80 anos (76,6%). Valores de PSA entre 4,1 a 10ng/mL foram mais frequentes. O escore de Gleason da biópsia revelou que 321 pacientes apresentavam tumor intermediário. Apenas 203 pacientes realizaram a prostectomina, e 61,5% das peças cirúrgicas também apresentaram tumor intermediário. Houve correlação significativa entre as faixas etárias e os níveis de PSA (R2=0,9319), e também entre o nível de PSA e os valores de escore Gleason da biópsia (p<0,05). Houve concordância entre os valores de escore de Gleason da biópsia com os da peça cirúrgica em 72,9% dos casos. CONCLUSÃO: Em nosso conhecimento, este foi o primeiro estudo epidemiológico de câncer de próstata na região do Vale do Aço. As informações fornecidas neste trabalho podem contribuir com programas para desenvolver ações de controle do câncer de próstata nesta região.
Palavras-Chave: carcinomas; prostate; neoplasms; diagnosis; diagnostic techniques; biopsy; epidemiology
. Adenocarcinoma prostático: análise clínica e epidemiológica. Revista da Sociedade Brasileira de Clínica Médica, v. 15, n. 03, p. 178-182, 2017. Disponível em: http://repositorio.ipen.br/handle/123456789/28615. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Análise da proína recombinante RPL-10 nativa expressa em sistema bacteriano. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO CIENTÍFICA, 18.; PROGRAMA DE BOLSAS E INICIAÇÃO CIENTÍFICA CNEN, 9.; PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO DESENVOLVIMENTO TECNOLÓGICO E INOVAÇÃO, 2., 24-25 de outubro, 2012, São Paulo, SP. Resumo expandido... 2012.
Palavras-Chave: proteins; bacteria; fluorescence spectroscopy; electrophoresis; animal cells; growth; genetic engineering
. Análise da proína recombinante RPL-10 nativa expressa em sistema bacteriano. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO CIENTÍFICA, 18.; PROGRAMA DE BOLSAS E INICIAÇÃO CIENTÍFICA CNEN, 9.; PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO DESENVOLVIMENTO TECNOLÓGICO E INOVAÇÃO, 2., 24-25 de outubro, 2012, São Paulo, SP. Resumo expandido... 2012. Disponível em: http://repositorio.ipen.br/handle/123456789/25906. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Caracterização do sítio catalítico da enzima conversora de angiotensina-I região N-domínio. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIACAO CIENTIFICA E TECNOLOGICA; SEMINARIO ANUAL PIBIC, 25.; SEMINARIO ANUAL PROBIC, 16.; SEMINARIO ANUAL PIBITI, 9, 6-7 de novembro, 2019, São Paulo, SP. Resumo expandido... São Paulo: IPEN-CNEN/SP, 2019. p. 100-101.
Palavras-Chave: angiotensin; enzymes; catalysts; proteins
. Caracterização do sítio catalítico da enzima conversora de angiotensina-I região N-domínio. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIACAO CIENTIFICA E TECNOLOGICA; SEMINARIO ANUAL PIBIC, 25.; SEMINARIO ANUAL PROBIC, 16.; SEMINARIO ANUAL PIBITI, 9, 6-7 de novembro, 2019, São Paulo, SP. Resumo expandido... São Paulo: IPEN-CNEN/SP, 2019. p. 100-101. Disponível em: http://repositorio.ipen.br/handle/123456789/30773. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Caracterização microbiológica de rejeitos radioativos provenientes do acidente de Goiânia. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO CIENTÍFICA; SEMINÁRIO ANUAL PIBIC, 29.; SEMINÁRIO ANUAL PROBIC, 20.; SEMINÁRIO ANUAL PIBITI, 13., 23-24 de novembro, 2023, São Paulo, SP. Resumo expandido... São Paulo, SP: IPEN-CNEN/SP, 2023.. Caracterização microbiológica de rejeitos radioativos provenientes do acidente de Goiânia. In: PROGRAMA INSTITUCIONAL DE BOLSAS DE INICIAÇÃO CIENTÍFICA; SEMINÁRIO ANUAL PIBIC, 29.; SEMINÁRIO ANUAL PROBIC, 20.; SEMINÁRIO ANUAL PIBITI, 13., 23-24 de novembro, 2023, São Paulo, SP. Resumo expandido... São Paulo, SP: IPEN-CNEN/SP, 2023. Disponível em: http://repositorio.ipen.br/handle/123456789/34305. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Estudo da expressao citoplasmica bacteriana de uma forma de prolactina humana e de sua solubilizacao e renaturacao a partir de corpos de inclusao. 2000. Tese (Doutoramento) - Instituto de Pesquisas Energeticas e Nucleares - IPEN/CNEN-SP, Sao Paulo. 105 p. Orientador: Paolo Bartolini.
Palavras-Chave: lth; escherichia coli; cytoplasm; bacteriophages; recombinant dna; separation processes; solubility; purification; hormones; genes; bacteria
. Estudo da expressao citoplasmica bacteriana de uma forma de prolactina humana e de sua solubilizacao e renaturacao a partir de corpos de inclusao. Orientador: Paolo Bartolini. 2000. 105 f. Tese (Doutoramento) - Instituto de Pesquisas Energeticas e Nucleares - IPEN/CNEN-SP, Sao Paulo. Disponível em: http://repositorio.ipen.br/handle/123456789/10835. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Evaluation of low doses of gamma irradiation in the formation of mineralization nodules in osteoblasts culture. In: ANAIS DA SOCIEDADE BRASILEIRA DE BIOCIENCIAS NUCLEARES, 09-11 de outubro, 2017, São Paulo, SP. Abstract... Rio de Janeiro, RJ: Sociedade Brasileira de Biociências Nucleares, 2017. p. 116-116. Abstract: Introduction: Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. The mineralized extracellular matrix is mainly composed of type I collagen, osteocalcin, and the inorganic mineral hydroxylapatite1. The use of radiation as therapy in some cancers causes great bone loss. However, low dose radiation may have the opposite effect. Low dose X-irradiation on osteoblastic culture had effects on proliferation and differentiation with increase of mineralization nodules2. However, there is little information on the potential therapeutic efficacy of low-dose gamma-irradiation in the formation of mineralization nodules. Objective: To evaluate the effects of irradiation with 60Co γ-rays in low doses in the formation of mineralization nodules in culture of osteoblasts. Methods: MC3T3-E1 cells were bought by the Banco de Células do Rio de Janeiro, Brazil (MC3T3-E1 Subclone 14). The cells were cultured in α-MEM medium consisting of 10% FBS and without β-glycerophosphate and L-ascorbic acid (GIBCO, Custom Product, Catalog No. A1049001) (Zhao Y, Guan H, Liu S et al. Biol. Pharm. Bull. 2005, 28(8):1371-1376). Plating efficiency assays: cells were plated at a density of 100 cell/plate into 60 mm Petri dishes. After 14 days the places were stained with violet crystal and the colonies were counted. -glycerophosphate and 50 mg/ml ascorbic acid, and analyzed on days 7, 14 and 21. Osteoblast culture irradiation assay: cells were plated at a density of 1x 105 cells/plate on 60 mm dishes and the next day were irradiated by 60Co source with 0 (as the control), 0.5, 1.0, 1.5 and 2.0 Gy using the GammaCell 220 – Irradiation Unit of Canadian-Atomic Energy Commission Ltd. (CTR-IPEN). On day 21 of culture, undifferentiated (without ascorbic acid and β-glycerophosphate), differentiating cells (0 Gy) and irradiated cells at different doses, the medium was removed, cells were washed with phosphate buffer saline, fixed with 70% ethyl alcohol and stained with Alizarin red S (Sigma). All in three biological replicates (a total of 54 samples) and multiple comparisons were assessed by One-way ANOVA followed by Bonferroni´s tests with GraphPad Prism version 6.0 software. P< 0.05 was considered statistically significant. Results: Plating efficiency (PF) analysis is generally considered to be the gold standard of assays for testing the sensitivity of cell lines to ionizing radiation or other cytotoxic agents in vitro. The results obtained were a PF of 30% for non-irradiated culture, however, the irradiated culture obtained 40% in relation to the no-irradiated one, already with 0.5 Gy, and this percentage was maintained in the other larger doses. Regarding the evaluation of the formation of mineralization nodules, significant difference in 0.5 Gy group was observed compared with the control group (0 Gy), 64.7±1.8 and 53.0±0.9, respectively. The groups of 1.0, 1.5 and 2.0 Gy obtained a decrease in the mineralization nodules. The data obtained with increasing irradiation produced an increase of mineralization nodules up to 0.5 Gy and in the higher doses had a decrease. Applying the data in a non-linear function it is observed that the line has a decreasing tendency with the negative angular coefficient. This analysis is in agreement with the hormesis model, in which low doses induce a stimulatory effect while high doses cause inhibition4. Conclusions: This study is one among the first that investigating the biophysics of low-dose gamma-irradiation on MC3T3-E1 culture, focusing on the potential applications in bone replacement therapy.. Evaluation of low doses of gamma irradiation in the formation of mineralization nodules in osteoblasts culture. In: ANAIS DA SOCIEDADE BRASILEIRA DE BIOCIENCIAS NUCLEARES, 09-11 de outubro, 2017, São Paulo, SP. Abstract... Rio de Janeiro, RJ: Sociedade Brasileira de Biociências Nucleares, 2017. p. 116-116. Disponível em: http://repositorio.ipen.br/handle/123456789/28823. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Expression of human prolactin in HEK293T using different transfection reagents. In: ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 46th, July 27-30, 2017, Águas de Lindóia, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular, 2017. Abstract: INTRODUCTION Prolactin is a hormone produced by the pituitary gland with numerous functions, such as lactation, reproduction, osmotic and immune regulation. This hormone is upregulated in cases of lack of lactation, infertility and cancer. Recombinant prolactin has been produced in Escherichia coli with an initial methionine which may cause immunological reactions, or in its authentic form in mammalian cells. Our laboratory has already synthesized human prolactin (hPRL) without initial methionine in E. coli periplasm and Chinese hamster ovary cells. CHO cells have been widely used in the synthesis of human recombinant proteins because of their similarity with human post-translational modifications as glycosylation. The HEK293, a human embryonic kidney cell, can do diverse glycosylation depend on culture conditions. OBJECTIVES This work compares different transfection reagents in the production of hPRL in HEK293T. MATERIALS AND METHODS The hPRL cDNA was inserted into the pEDdc vector donated by the Genetics Institute, USA. Three transfection reagents were used: LipofectamineTM (Thermo), XfectTM (Clontech), and ExpiFectamineTM (Thermo). HEK293T cells, a human strain, were cultured in 10 cm² Ø petri dishes with RPMI 1640 medium with 10% fetal bovine serum (FBS). After transfection, the medium was changed to serum free CHO-S-SFM II (Invitrogen, USA). 100% of the medium was collected and changed every two days. The collected medium was stored at -80°C. Samples were analyzed by SDS-PAGE, Western blotting and HPLC. DISCUSSION AND RESULTS The glycosylated and non-glycosylated hPRL forms secreted into the culture medium were confirmed by Western blot and RPHPLC in the three transfected cultures in recombinant human cells. The reagent with the best result was Xfect (2 μg/mL), followed by Lipofectamine (1.6 μg/mL) and Expifectamine (1.2 μg/mL). CONCLUSION The transient expression of hPRL using HEK293T cells enable laboratory production of glycosylated hPRL for future studies of N-glycans produced by these cells.. Expression of human prolactin in HEK293T using different transfection reagents. In: ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 46th, July 27-30, 2017, Águas de Lindóia, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular, 2017. Disponível em: http://repositorio.ipen.br/handle/123456789/28436. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Gene analysis in patients with premature ovarium failure or gonadal dysgenesis: A preliminary study. Maturitas, v. 57, n. 4, p. 399-404, 2007.
Palavras-Chave: genes; ovaries; failures; gene mutations; gonads
. Gene analysis in patients with premature ovarium failure or gonadal dysgenesis: A preliminary study. Maturitas, v. 57, n. 4, p. 399-404, 2007. Disponível em: http://repositorio.ipen.br/handle/123456789/7817. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Genetic diversity of Pneumocystis jirovecii from a cluster of cases of pneumonia in renal transplant patients: Cross-sectional study. Mycoses, v. 61, n. 11, p. 845-852, 2018. DOI: 10.1111/myc.12823 Abstract: Pneumocystis jirovecii can cause severe potentially life-threatening pneumonia (PCP) in kidney transplant patients. Prophylaxis of patients against PCP in this setting is usually performed during 6 months after transplantation. The aim of this study is to describe the molecular epidemiology of a cluster of PCP in renal transplant recipients in Brazil. Renal transplant patients who developed PCP between May and December 2011 had their formalin-fixed paraffin-embedded (FFPE) lung biopsy samples analysed. Pneumocystis jirovecii 23S mitochondrial large subunit of ribosomal RNA (23S mtLSU-rRNA), 26S rRNA, and dihydropteroate synthase (DHPS) genes were amplified by polymerase chain reaction (PCR), sequenced, and analysed for genetic variation. During the study period, 17 patients developed PCP (only four infections were documented within the first year after transplantation) and six (35.3%) died. Thirty FFPE samples from 11 patients, including one external control HIV-infected patient, had fungal DNA successfully extracted for further amplification and sequencing for all three genes. A total of five genotypes were identified among the 10 infected patients. Of note, four patients were infected by more than one genotype and seven patients were infected by the same genotype. DNA extracted from FFPE samples can be used for genotyping; this approach allowed us to demonstrate that multiple P. jirovecii strains were responsible for this cluster, and one genotype was found infecting seven patients. The knowledge of the causative agents of PCP may help to develop new initiatives for control and prevention of PCP among patients undergoing renal transplant and improve routine PCP prophylaxis.
Palavras-Chave: kidneys; transplants; pneumonia; diseases; genetics; epidemiology; patients; immune system diseases
. Genetic diversity of Pneumocystis jirovecii from a cluster of cases of pneumonia in renal transplant patients: Cross-sectional study. Mycoses, v. 61, n. 11, p. 845-852, 2018. DOI: 10.1111/myc.12823. Disponível em: http://repositorio.ipen.br/handle/123456789/29341. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. High production and optimization of the method for obtaining pure recombinant human prolactin. Protein Expression and Purification, v. 152, p. 131-136, 2018. DOI: 10.1016/j.pep.2018.07.015 Abstract: Prolactin is a pituitary hormone that is involved diverse physiological functions, such as lactation, reproduction, metabolism, osmoregulation, immunoregulation, and behavior. Its level of glycosylation is low in vivo, which favors its expression in bacterial systems. In the present work recombinant human prolactin (rec-hPRL) was expressed from the p1813-hPRL vector in Escherichia coli strain in inclusion bodies with 530.67 mg of rec-hPRL per liter of induced bacterial culture. The solubilization and renaturation of rec-hPRL followed by two methods described in the literature for this protein: one with detergent and basic pH, and other urea and dialyses was done by studying. The protocol with detergent/basic pH was not successful, whereas protocol with urea/dialyses was obtained pure protein and this was optimized. Rec-hPRL was obtained in a soluble, pure and active form, when the sample was 8-fold concentrated in the solubilization phase, allowing 33% recovery, 3-fold more that the original method. The pure protein was obtained with 38.37 i. u./mg activity, which is three times greater than that of the PRL standard from the WHO. In conclusion, this work obtained the highest production of rechPRL, and concentrating the sample eight times in the solubilization stage was decisive for obtaining a highly concentrated, active protein for future work.
Palavras-Chave: lth; hormones; biological effects; bacteria; proteins; in vitro; bioassay; statistical data; lactogens; pituitary hormones; biotechnology
. High production and optimization of the method for obtaining pure recombinant human prolactin. Protein Expression and Purification, v. 152, p. 131-136, 2018. DOI: 10.1016/j.pep.2018.07.015. Disponível em: http://repositorio.ipen.br/handle/123456789/29259. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. In vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake Crotalus durissus. Journal of Antibiotics, v. 64, n. 4, p. 327-331, 2011.
Palavras-Chave: antimicrobial agents; peptides; hemolysis; escherichia coli; snakes; venoms; toxins
. In vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake Crotalus durissus. Journal of Antibiotics, v. 64, n. 4, p. 327-331, 2011. Disponível em: http://repositorio.ipen.br/handle/123456789/4426. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Influence of the expression vector and its elements on recombinant human prolactin synthesis in Escherichia coli; co-directional orientation of replication and transcription is highly critical. Journal of Microbiological Methods, v. 191, p. 1-8, 2021. DOI: 10.1016/j.mimet.2021.106340 Abstract: The aim of the present work was to define a bacterial expression system that is particularly efficient for the synthesis of recombinant human prolactin (hPRL). In previous work, based on experiments that were basically carried out in parallel with the present ones, the synthesis of rec-hPRL by the p1813-hPRL vector in E. coli HB2151 was >500 mg/L, while it was much lower here (2.5–4-fold), in the RB791 and RRI strains. The highest positive influence on rec-hPRL synthesis was due to the transcription-replication co-orientation of hPRL cDNA and the ori/antibiotic resistance gene, responsible for up to a ~ 5–6-fold higher expression yield. In conclusion, this work confirmed that each bacterial strain of E. coli has a genetic background that can allow a different level of heterologous protein synthesis. The individual study of each element indicated that its action critically depends on the reading orientation in which it is located inside the vector: co-directional orientation of replication and transcription, in fact, greatly increased the level of rec-hPRL expression
Palavras-Chave: lth; escherichia coli; collisions; plasmids; dna
. Influence of the expression vector and its elements on recombinant human prolactin synthesis in Escherichia coli; co-directional orientation of replication and transcription is highly critical. Journal of Microbiological Methods, v. 191, p. 1-8, 2021. DOI: 10.1016/j.mimet.2021.106340. Disponível em: http://repositorio.ipen.br/handle/123456789/32515. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A new approach for purification of the catalytic site of the Angiotensin Conversion Enzyme, N domain, mediated by the ELP-Inten system. Biophysical Reviews, v. 13, n. 6, p. 1305-1305, 2021. DOI: 10.1007/s12551-021-00845-2 Abstract: INTRODUCTION Angiotensin-converting enzyme I, ACE, is a key part of the renin-angiotensin system whose main function is to regulate blood pressure and balance of salts in the body. ACE1 has two isoforms, somatic, sACE, and testicular, tACE. sACE possesses two domains, N- C-, with catalytic sites which exhibit 60% sequence identity. These domains differ in terms of chloride-ion activation profiles, rates of peptide hydrolysis and sensitivities to various inhibitors. N-domain has specific action in the hydrolyze of Alzheimer’s diseases beta amyloid bodies and angiotensin 1-7, which active the MAS receptor and triggering anti-thrombotic and anti-inflammatory actions. OBJECTIVES The objective this work was to obtain catalytic site Ala361 to Gli468 of the N-domain region, csACEN, isolation without chromatographic and denaturant chemical process. MATERIALS AND METHODS For that, a new methodology was used in the expression of the csACEN peptide, in which the peptide was linked to the elastin-like polypeptide, ELP, and Intein, and expressed at 37C. The characterization of catalytic site was made by SDS-PAGE and dot blotting. DISCUSSION AND RESULTS The culture temperature at 37C significantly increased the expression of the ELP/Intein/csACEN fusion protein. This culture was lysed at a low temperature allowing the fusion protein to become soluble. The precipitation of ELP at high concentrations of ammonium sulfate were obtained in 0.57 M and 0.8 M. Intein autocleavage occurs at acidic pH and it is important to pay attention to: pI 6.65 for csACEN and pI 6.87 for ELPcsACEN, which are very low. The best autocleavage efficiency was withMES and TriHCl buffers, pH 6.3 and 6.8, respectively, in which pure csACEn peptide was obtained. CONCLUSION The strategy used to obtain the Ala361 to Gli468 catalytic site in soluble and pure form was obtained with success and the protocol for obtaining similar peptides was established.. A new approach for purification of the catalytic site of the Angiotensin Conversion Enzyme, N domain, mediated by the ELP-Inten system. Biophysical Reviews, v. 13, n. 6, p. 1305-1305, 2021. DOI: 10.1007/s12551-021-00845-2. Disponível em: http://repositorio.ipen.br/handle/123456789/32883. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A new approach for purification of the catalytic site of the Angiotensin Conversion Enzyme, N domain, mediated by the ELP-Inten system. In: CONGRESS OF THE INTERNATIONAL UNION FOR PURE APPLIED BIOPHYSICS, 20th; ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 50th; CONGRESS OF BRAZILIAN BIOPHYSICS SOCIETY, 45th; BRAZILIAN SOCIETY ON NUCLEAR BIOSCIENCES CONGRESS, 13th, October 4-8, 2021, São Paulo, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular (SBBq), 2021. p. 143-143. Abstract: Angiotensin-converting enzyme I, ACE, is a key part of the renin-angiotensin system whose main function is to regulate blood pressure and balance of salts in the body. ACE1 has two isoforms, somatic, sACE, and testicular, tACE. sACE possesses two domains, N- C-, with catalytic sites which exhibit 60% sequence identity. These domains differ in terms of chloride-ion activation profiles, rates of peptide hydrolysis and sensitivities to various inhibitors. N-domain has specific action in the hydrolyze of Alzheimer’s diseases beta amyloid bodies and angiotensin 1-7, which active the MAS receptor and triggering anti-thrombotic and anti-inflammatory actions. The objective this work was to obtain catalytic site Ala361 to Gli468 of the N-domain region, csACEN, isolation without chromatographic and denaturant chemical process. For that, a new methodology was used in the expression of the csACEN peptide, in which the peptide was linked to the elastin-like polypeptide, ELP, and Intein, and expressed at 37C. The characterization of catalytic site was made by SDS-PAGE and dot blotting. The culture temperature at 37C significantly increased the expression of the ELP/Intein/csACEN fusion protein. This culture was lysed at a low temperature allowing the fusion protein to become soluble. The precipitation of ELP at high concentrations of ammonium sulfate were obtained in 0.57 M and 0.8 M. Intein autocleavage occurs at acidic pH and it is important to pay attention to: pI 6.65 for csACEN and pI 6.87 for ELPcsACEN, which are very low. The best autocleavage efficiency was with MES and TriHCl buffers, pH 6.3 and 6.8, respectively, in which pure csACEn peptide was obtained. The strategy used to obtain the Ala361 to Gli468 catalytic site in soluble and pure form was obtained with success and the protocol for obtaining similar peptides was established.
Palavras-Chave: angiotensin; enzymes; inflammation; thrombosis; temperature dependence
. A new approach for purification of the catalytic site of the Angiotensin Conversion Enzyme, N domain, mediated by the ELP-Inten system. In: CONGRESS OF THE INTERNATIONAL UNION FOR PURE APPLIED BIOPHYSICS, 20th; ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 50th; CONGRESS OF BRAZILIAN BIOPHYSICS SOCIETY, 45th; BRAZILIAN SOCIETY ON NUCLEAR BIOSCIENCES CONGRESS, 13th, October 4-8, 2021, São Paulo, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular (SBBq), 2021. p. 143-143. Disponível em: http://repositorio.ipen.br/handle/123456789/32867. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A new approach for purification of the catalytic site of the angiotensin-conversion enzyme, N-domain, mediated by the ELP-Intein system. Journal of Pharmacological and Toxicological Methods, v. 116, p. 1-6, 2022. DOI: 10.1016/j.vascn.2022.107174 Abstract: Angiotensin-converting enzyme I (ACE) is a key part of the renin-angiotensin system. Its main function is to regulate blood pressure and the balance of salts in the body. Somatic ACE has two domains, N-C-, each of which has a catalytic site that exhibits 60%sequence identity. The N-domain has a specific action in the hydrolysis of beta-amyloid bodies and angiotensin (1–7), which activates the MAS receptor and triggers anti-thrombotic and anti-inflammatory actions. Our goal was to obtain the catalytic site Ala361 to Gly468 of the N domain region, csACEN, without needing purification by chromatography. We employed a method that uses an Elastin-like Polypeptide (ELP) and Intein sequences linked to the peptide of interest. The more differential for obtaining the pure peptide was the cultivation temperatures in the synthesis of ELPcsACEN at 37 °C, with a significant increase in expression. In the purification by ELP precipitation, we recorded the highest efficiency in the concentrations of 0.57 M and 0.8 M of ammonium sulfate buffer. Intein autocleavage study allows removal of the ELP sequence at acidic pH, with the buffers MES and Tris-HCl The present study defined the best conditions for obtaining pure csACEN that the literature has not yet described for peptides. Obtaining pure csACEN aims at future studies for therapeutic use in hypertension, Alzheimer's, and oncology.
Palavras-Chave: angiotensin; enzymes; domain structure; polypeptides
. A new approach for purification of the catalytic site of the angiotensin-conversion enzyme, N-domain, mediated by the ELP-Intein system. Journal of Pharmacological and Toxicological Methods, v. 116, p. 1-6, 2022. DOI: 10.1016/j.vascn.2022.107174. Disponível em: http://repositorio.ipen.br/handle/123456789/33431. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A new approach to obtain the catalytic site of human Angiotensin Converting Enzyme. In: EUROPEAN SYMPOSIUM ON BIOCHEMICAL ENGINEERING SCIENCES, 12th, September 9-12, 2018, Lisbon, Portugal. Abstract... Frankfurt am Main, Germany: European Society of Biochemical Engineering Sciences - ESBES, 2018. Abstract: Angiotensin-converting enzyme I (ACE) is a key part of the renin-angiotensin system whose main function is to regulate blood pressure. The sACE possesses two domains, N- C-, with catalytic sites which exhibit 60% sequence identity. These domains differ in terms of chloride-ion activation profiles, rates of peptide hydrolysis of angiotensin I, bradykinin, angiotensina (1-7), beta-amyloid peptide and sensitivities to various inhibitors. A more detailed analysis shows that these regions are composed of HEMGH and EAIGD sequences, which are the catalytic sites. Our question is: If the synthesis of catalytic sites with corrects structure and activity could be a good model per si to study new drugs. In our laboratory the catalytic site of the C-domain was obtained with correct structural conformation and with enzymatic activity. The objective this work is to obtain the Ala361 to Gli468 catalytic site, N-domain, in a structural conformation that resembles its native form. The 380 pb cDNA to catalytic site was cloned in the pE1 vector (kindly provided by Dr. David Wood), elastinlike-polyptide (ELP) tag sequence was linked with catalytic site, ELP~csACEn recombinant protein, this was expressed in bacteria with Terrificus broth with 0.1 mM IPTG for 20h. Harvested cells were resuspended in TE buffer, after sonication and centrifugation the pellet was resuspended the same buffer. The ELP~csACEn was precipitated with 0.8 M ammonium sulfate and the tag sequence was cleaved by pH change, pH 6.2. All steps were analyzed by SDS-PAGE, Dot and Western blotting. The catalytic site was synthesized from bacterial system with ELP sequence tag in soluble form. The purification process was done by ammonium sulfate precipitation and cleavage of the ELP sequence by changing to acidic pH. The characterization of catalytic site by SDS-PAGE shows that this is pure and Western blotting immunological assay confirmed the identity of the protein as csACEn. The strategy used to obtain the Ala361 to Gli468 catalytic site in soluble and pure form was successful. The next steps: we will continue with the Maldi-tof and structural conformation analyzes.. A new approach to obtain the catalytic site of human Angiotensin Converting Enzyme. In: EUROPEAN SYMPOSIUM ON BIOCHEMICAL ENGINEERING SCIENCES, 12th, September 9-12, 2018, Lisbon, Portugal. Abstract... Frankfurt am Main, Germany: European Society of Biochemical Engineering Sciences - ESBES, 2018. Disponível em: http://repositorio.ipen.br/handle/123456789/30194. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A new approach to obtain the catalytic sites region of human sACE with correct fold and activity. Journal of Biotechnology and Biomaterials, v. 7, n. 1, p. 96-96, 2017. DOI: 10.4172/2155-952X.C1.071 Abstract: Angiotensin-converting enzyme I (ACE) is a membrane-bound that catalyzes the conversion of angiotensin I to the potent vasopressor angiotensin II. ACE is a key part of the renin-angiotensin system, which regulates blood pressure and is widely distributed throughout the body. There are two isoforms of human ACE, including the somatic ACE (sACE) present in somatic tissue and the testicular ACE (tACE) present in male germinal cells. The sACE possesses two domains, N- C- domains, with catalytic sites which exhibit 60% sequence identity. These domains differ in terms of chloride-ion activation profiles, rates of peptide hydrolysis of angiotensin I, bradykinin, Goralatide, Luliberin, substance P, angiotensina, beta-amyloid peptide and sensitivities to various inhibitors. A more detailed analysis shows that these regions are composed of HEMGH and EAIGD sequences that bind zinc ions to facilitate catalytic activity (Fig. 1). Our question is: If the synthesis of catalytic sites with corrects structure and activity could be a good model per si to study new drugs. The objective was to obtain the Ala361 a Gli468 and Ala959 to Ser1066 catalytic regions sACE in a structural conformation that resembles its native form. The catalytic regions were obtained from bacterial system; the expression of this protein in soluble form enables completion of the solubilization/purification steps without the need for refolding. The characterization of Ala959 to Ser1066 region shows that this has an α-helix and β-strand structure, Fig. 1b, which zinc ion (essential for its activity) binds to, and with enzymatic activity. Our conclusion is that the strategy used to obtain the Ala959 to Ser1066 region in the correct structural conformation and with activity was successful.
Palavras-Chave: angiotensin; enzyme inhibitors; zinc ions; bacteria
. A new approach to obtain the catalytic sites region of human sACE with correct fold and activity. Journal of Biotechnology and Biomaterials, v. 7, n. 1, p. 96-96, 2017. DOI: 10.4172/2155-952X.C1.071. Disponível em: http://repositorio.ipen.br/handle/123456789/31166. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Optimization of the process of expression in E. coli and purification of the catalytic sites of the ACE1 by the ELP-Intein system. In: ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY (SBBq), 51st; CONGRESS OF BRAZILIAN BIOPHYSICAL SOCIETY (SBBf)/LATIN AMERICAN FEDERATION OF BIOPHYSICAL SOCIETIES (Lafebs), 46th, September 5-8, 2022, Águas de Lindóia, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular - SBBq, 2022. p. 220-220. Abstract: INTRODUCTION: Angiotensin I-converting enzyme (ACE) is a fundamental part of the renin-angiotensin system; this has two domains, N- and C-, each of which has a catalytic site that exhibits 60% sequence identity. Its actions are in the control of blood pressure, protection of the brain by cleavage of beta-amyloid bodies, cell proliferation, formation of hematopoietic stem cells, among others. OBJECTIVES: Obtaining the catalytic sites Ala361 to Gly468 (N domain region, csACEN) and Ala959 to Ser1066 (C domain region, csACEC) in pure form and with their correct structural conformation. MATERIALS AND METHODS: Expression conditions of pE1csACEN and pE1csACEC vectors in E. coli BL21(DE3) strain: cultures grown in Terrific Broth at 37⁰C at 140 rpm for 20–24 h and 0.1 mM IPTG. Purification by Elastin-like Polypeptide (ELP) precipitation: ELP-bound catalytic sites were purified with two ammonium sulfate precipitations (ASp). Remotion of ELP: by autocleavage of the Intein sequence using the buffers: sodium phosphate, sodium cacodylate, MES and Tris-HCl. The ELP/Intein was removed from the sample by ASp. The analyzes of all stages of the process were performed by SDS-PAGE and Dot blotting. DISCUSSION AND RESULTS: The differential for obtaining the pure peptides was the temperature of 37⁰C, with a significant increase in expression concerning the cultivation of 16⁰C. In the ELP purification steps, ammonium sulfate buffer concentrations of 0.57 M and 0.8 M were the most efficient. Intein's self-cleaving was more efficient with MES buffers and Tris-HCl for ELPsACEN and ELPsACEC, respectively. Structural analysis by Circular Dichroism and Fluorescence confirmed the correct structure of the pure peptides. CONCLUSION: In the present work, we defined the most efficient conditions for expression, purification, and obtaining of ACE catalytic sites in pure form. The csACEN and csACEC peptides will allow greater assertiveness in obtaining and characterizing new hypertensive drugs and in the hydrolysis of substrates such as beta-amyloid.. Optimization of the process of expression in E. coli and purification of the catalytic sites of the ACE1 by the ELP-Intein system. In: ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY (SBBq), 51st; CONGRESS OF BRAZILIAN BIOPHYSICAL SOCIETY (SBBf)/LATIN AMERICAN FEDERATION OF BIOPHYSICAL SOCIETIES (Lafebs), 46th, September 5-8, 2022, Águas de Lindóia, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular - SBBq, 2022. p. 220-220. Disponível em: http://repositorio.ipen.br/handle/123456789/33911. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Periplasmic synthesis and purification of the human prolactin antagonist Δ1‑11‑G129R‑hPRL. AMB Express, v. 11, n. 1, p. 1-12, 2021. DOI: 10.1186/s13568-021-01209-5 Abstract: The human prolactin antagonist Δ1-11-G129R-hPRL is a 21.9 kDa recombinant protein with 188 amino acids that downregulates the proliferation of a variety of cells expressing prolactin receptors. Periplasmic expression of recombinant proteins in E. coli has been considered an option for obtaining a soluble and correctly folded protein, as an alternative to cytoplasmic production. The aim of this work was, therefore, to synthesize for the first time, the Δ1-11-G129R-hPRL antagonist, testing different activation temperatures and purifying it by classical chromatographic techniques. E. coli BL21(DE3) strain was transformed with a plasmid based on the pET25b( +) vector, DsbA signal sequence and the antagonist cDNA sequence. Different doses of IPTG were added, activating under different temperatures, and extracting the periplasmic fluid via osmotic shock. The best conditions were achieved by activating at 35 °C for 5 h using 0.4 mM IPTG, which gave a specific expression of 0.157 ± 0.015 μg/mL/A600 at a final optical density of 3.43 ± 0.13 A600. Purification was carried out by nickel-affinity chromatography followed by size-exclusion chromatography, quantification being performed via high-performance size-exclusion chromatography (HPSEC). The prolactin antagonist was characterized by SDS-PAGE, Western blotting, reversed-phase high-performance liquid chromatography (RP-HPLC) and MALDI-TOF–MS. The final product presented > 95% purity and its antagonistic effects were evaluated in vitro in view of potential clinical applications, including inhibition of the proliferation of cancer cells overexpressing the prolactin receptor and specific antidiabetic properties, taking also advantage of the fact that this antagonist was obtained in a soluble and correctly folded form and without an initial methionine.
Palavras-Chave: lth; peptides; chromatography; neoplasms; purification; mass spectra; bioassay; plasma
. Periplasmic synthesis and purification of the human prolactin antagonist Δ1‑11‑G129R‑hPRL. AMB Express, v. 11, n. 1, p. 1-12, 2021. DOI: 10.1186/s13568-021-01209-5. Disponível em: http://repositorio.ipen.br/handle/123456789/31998. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Refolding of endostatin from inclusion bodies using high hydrostatic pressure. Analytical Biochemistry, v. 379, n. 1, p. 32-39, 2008.
Palavras-Chave: escherichia coli; proteins; hydrostatics; bacteria; inclusions; agglomeration; collagen; endothelium
. Refolding of endostatin from inclusion bodies using high hydrostatic pressure. Analytical Biochemistry, v. 379, n. 1, p. 32-39, 2008. Disponível em: http://repositorio.ipen.br/handle/123456789/4982. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
Itens para a visualização no momento 1-20 de 26
Buscar no repositório
Navegar
Minha conta
Visualizar
A pesquisa no RD utiliza os recursos de busca da maioria das bases de dados. No entanto algumas dicas podem auxiliar para obter um resultado mais pertinente.
✔ É possível efetuar a busca de um autor ou um termo em todo o RD, por meio do Buscar no Repositório , isto é, o termo solicitado será localizado em qualquer campo do RD. No entanto esse tipo de pesquisa não é recomendada a não ser que se deseje um resultado amplo e generalizado.
✔ A pesquisa apresentará melhor resultado selecionando um dos filtros disponíveis em Navegar
✔ Os filtros disponíveis em Navegar tais como: Coleções, Ano de publicação, Títulos, Assuntos, Autores, Revista, Tipo de publicação são autoexplicativos. O filtro, Autores IPEN apresenta uma relação com os autores vinculados ao IPEN; o ID Autor IPEN diz respeito ao número único de identificação de cada autor constante no RD e sob o qual estão agrupados todos os seus trabalhos independente das variáveis do seu nome; Tipo de acesso diz respeito à acessibilidade do documento, isto é , sujeito as leis de direitos autorais, ID RT apresenta a relação dos relatórios técnicos, restritos para consulta das comunidades indicadas.

A opção Busca avançada utiliza os conectores da lógica boleana, é o melhor recurso para combinar chaves de busca e obter documentos relevantes à sua pesquisa, utilize os filtros apresentados na caixa de seleção para refinar o resultado de busca. Pode-se adicionar vários filtros a uma mesma busca.
Exemplo:
Buscar os artigos apresentados em um evento internacional de 2015, sobre loss of coolant, do autor Maprelian.
Autor: Maprelian
Título: loss of coolant
Tipo de publicação: Texto completo de evento
Ano de publicação: 2015
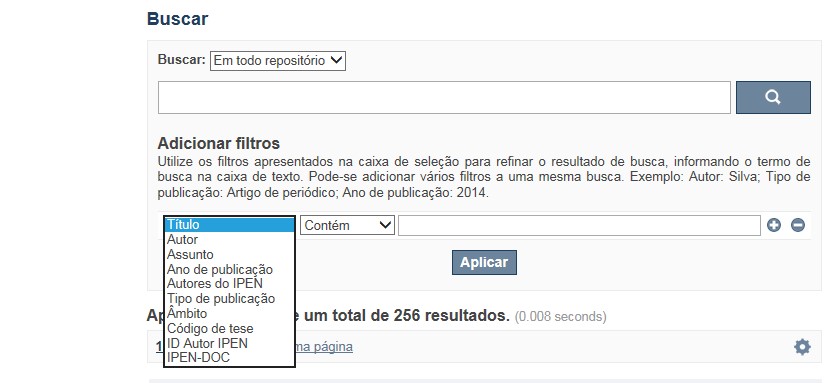
✔ Para indexação dos documentos é utilizado o Thesaurus do INIS, especializado na área nuclear e utilizado em todos os países membros da International Atomic Energy Agency – IAEA , por esse motivo, utilize os termos de busca de assunto em inglês; isto não exclui a busca livre por palavras, apenas o resultado pode não ser tão relevante ou pertinente.
✔ 95% do RD apresenta o texto completo do documento com livre acesso, para aqueles que apresentam o ![]() significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
✔ Ao efetuar a busca por um autor o RD apresentará uma relação de todos os trabalhos depositados no RD. No lado direito da tela são apresentados os coautores com o número de trabalhos produzidos em conjunto bem como os assuntos abordados e os respectivos anos de publicação agrupados.
✔ O RD disponibiliza um quadro estatístico de produtividade, onde é possível visualizar o número dos trabalhos agrupados por tipo de coleção, a medida que estão sendo depositados no RD.
✔ Na página inicial nas referências são sinalizados todos os autores IPEN, ao clicar nesse símbolo ![]() será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
✔ Na página do pesquisador, é possível verificar, as variações do nome, a relação de todos os trabalhos com texto completo bem como um quadro resumo numérico; há links para o Currículo Lattes e o Google Acadêmico ( quando esse for informado).
ATENÇÃO!
ESTE TEXTO "AJUDA" ESTÁ SUJEITO A ATUALIZAÇÕES CONSTANTES, A MEDIDA QUE NOVAS FUNCIONALIDADES E RECURSOS DE BUSCA FOREM SENDO DESENVOLVIDOS PELAS EQUIPES DA BIBLIOTECA E DA INFORMÁTICA.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
1. Portaria IPEN-CNEN/SP nº 387, que estabeleceu os princípios que nortearam a criação do RDI, clique aqui.
2. A experiência do Instituto de Pesquisas Energéticas e Nucleares (IPEN-CNEN/SP) na criação de um Repositório Digital Institucional – RDI, clique aqui.
O Repositório Digital do IPEN é um equipamento institucional de acesso aberto, criado com o objetivo de reunir, preservar, disponibilizar e conferir maior visibilidade à Produção Científica publicada pelo Instituto, desde sua criação em 1956.
Operando, inicialmente como uma base de dados referencial o Repositório foi disponibilizado na atual plataforma, em junho de 2015. No Repositório está disponível o acesso ao conteúdo digital de artigos de periódicos, eventos, nacionais e internacionais, livros, capítulos, dissertações, teses e relatórios técnicos.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
