Navegação IPEN por Autores IPEN "NANDENHA, JULIO"
- Página inicial
- →
- IPEN
- →
- Navegação IPEN por Autores IPEN
- Sobre
- Perfil Técnico
- Política de funcionamento
- Ajuda
- Apresentação
Navegação IPEN por Autores IPEN "NANDENHA, JULIO"
Itens para a visualização no momento 1-18 de 18
-
. Borohydride reduction method for PdIn/C electrocatalysts synthesis towards glycerol electrooxidation under alkaline condition. Electroanalysis, v. 33, n. 4, p. 1115-1120, 2021. DOI: 10.1002/elan.202060322 Abstract: Pd−In/C electrocatalysts were synthesized by the adapted borohydride reduction method in different atomic ratios. Electrocatalysts were evaluated by conventional electrochemical techniques and direct glycerol fuel cells. X-ray diffraction profiles indicated the structure of Pd and In (fcc) phases, as well as the presence of In higher oxidation states. Regarding Transmission electron microscopy, it showed the particle‘s average diameters between 6.1–12.7 nm. All PdIn/C electrocatalysts showed high current values for −0.30 V vs. Ag/AgCl, which the best one was PdIn/C 90 : 10. Higher performance for glycerol oxidation was observed in polarization curves at 90 °C for PdIn/C (30 : 70) composition.
Palavras-Chave: borohydrides; glycerol; alkaline electrolyte fuel cells; fuel cells; oxidation; polarization
. Borohydride reduction method for PdIn/C electrocatalysts synthesis towards glycerol electrooxidation under alkaline condition. Electroanalysis, v. 33, n. 4, p. 1115-1120, 2021. DOI: 10.1002/elan.202060322. Disponível em: http://repositorio.ipen.br/handle/123456789/32011. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Carbon supported hybrids nanostructures PtSn with CeO2 nanorods for Direct Ethanol Fuel Cells. In: REUNIAO ANUAL DA SOCIEDADE BRASILEIRA DE QUIMICA: CONSTRUINDO O AMANHA, 41., 21-24 de maio, 2018, Foz do Iguaçu, PR. Resumo... São Paulo: Sociedade Brasileira de Química, 2018. Abstract: New energetic sources have been the focus of current researches, including on fuel cells. This device has as its operating principle the conversion of chemical energy into electrical energy, making possible the use of renewable energy sources [1]. In the fuel cells operation are applied nanostructured electrocatalysts capable of oxidizing organic molecules such as ethanol [2]. This work was carried out using PtSn-based electrocatalysts with ceria nanorods (CeO2), synthesized by chemical reduction method via sodium borohydride [2], and supported on carbon Vulcan XC 72 (20% w/w). The synthesized materials have the following mass metal ratios: Pt/C; Pt3Sn1/ C and Pt3Sn1_20%NR/ C, with % of the Pt3Sn1 load replaced by ceria nanorods in the last nanomaterial, in order to reduce the costs of noble metals such as platinum. The evaluation of the activity for ethanol oxidation is given by polarization and power density curves, according to Figure 1. From the polarization curve it was observed that the Pt3Sn1_20%NR/ presented higher open circuit potential value (572 mV), and power density higher than the Pt/C composite material. The Pt3Sn1/C material presented a higher value of power density related to the others, but Pt3Sn1_20%NR/C generated a power density for ethanol oxidation relatively close to the best activity material, by the supply of oxygenated species improved the electrocatalytic activity for CO oxidation, making its application possible.. Carbon supported hybrids nanostructures PtSn with CeO2 nanorods for Direct Ethanol Fuel Cells. In: REUNIAO ANUAL DA SOCIEDADE BRASILEIRA DE QUIMICA: CONSTRUINDO O AMANHA, 41., 21-24 de maio, 2018, Foz do Iguaçu, PR. Resumo... São Paulo: Sociedade Brasileira de Química, 2018. Disponível em: http://repositorio.ipen.br/handle/123456789/29719. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. cis-[6-(pyridin-2-yl)-1,3,5-triazine-2,4-diamine](dichloride) palladium(II)-based electrolyte membrane reactors for partial oxidation methane to methanol. ACS Omega, v. 7, n. 28, p. 24249-24255, 2022. DOI: 10.1021/acsomega.2c01463 Abstract: Methane is an abundant resource and the main constituent of natural gas. It can be converted into higher value-added products and as a subproduct of electricity co-generation. The application of polymer electrolyte reactors for the partial oxidation of methane to methanol to co-generate power and chemical products is a topic of great interest for gas and petroleum industries, especially with the use of materials with a lower amount of metals, such as palladium complex. In this study, we investigate the ideal relationship between cis-[6-(pyridin-2-yl)-1,3,5-triazine-2,4-diamine(dichloride)palladium(II)] (Pd-complex) nanostructure and carbon to obtain a stable, conductive, and functional reagent diffusion electrode. The physical and structural properties of the material were analyzed by Fourier transform infrared (FT-IR) and Raman spectroscopies, transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques. The electrocatalytic activity studies revealed that the most active proportion was 20% of Pd-complex supported on carbon (m/m), which was measured with lower values of open-circuit and power density but with higher efficiency in methanol production with reaction rates of r = 4.2 mol L–1·h–1 at 0.05 V.
Palavras-Chave: electrolytes; fuel cells; membranes; methane; oxidation; electrocatalysts
. cis-[6-(pyridin-2-yl)-1,3,5-triazine-2,4-diamine](dichloride) palladium(II)-based electrolyte membrane reactors for partial oxidation methane to methanol. ACS Omega, v. 7, n. 28, p. 24249-24255, 2022. DOI: 10.1021/acsomega.2c01463. Disponível em: http://repositorio.ipen.br/handle/123456789/33361. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. CO2 reduction on Cu/C used as a cathode in a polymeric electrolyte reactor: fuel cell type. International Journal of Hydrogen Energy, v. 47, n. 6, p. 4010-4017, 2022. DOI: 10.1016/j.ijhydene.2021.11.008 Abstract: CO2 is one of the leading greenhouse gases, so studies that turn this gas into higher value-added products, that function as simpler and cheaper hydrogen stores, as an alcohols, are extremely important. In this work we using a polymeric Electrolyte Reactor– fuel cell type supplied with H2 on platinum anode and dry CO2 in the cathode with a copper-carbon electrocatalyst. Copper nanoparticles supported on carbon Vulcan XC72 were produced by the sodium borohydride reduction method. The XRD revealed the presence of two different phases, CuO and Cu2O. In addition, the TEM images revealed agglomerates presence. The water, formaldehyde, methanol, methane, formic acid, dimethyl ether, oxalic acid, dimethyl carbonate, and ethylene-glycol were observed by differential mass spectroscopy on line with the reaction and the onset potential for each product and these results were confirmed by infrared spectroscopy – ATR-FTIR setup. This work showed the mapping CO2 reduced compounds for onset potential proposing some contributions to the literature.
Palavras-Chave: fuel cells; carbon dioxide; reduction; polymerization; electrolytes; copper
. CO2 reduction on Cu/C used as a cathode in a polymeric electrolyte reactor: fuel cell type. International Journal of Hydrogen Energy, v. 47, n. 6, p. 4010-4017, 2022. DOI: 10.1016/j.ijhydene.2021.11.008. Disponível em: http://repositorio.ipen.br/handle/123456789/32994. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Comparison of various atomic compositions of Au@Pd/C, Pd/C, and AuPd/C electrocatalysts for direct ethanol fuel cells. Energy Storage, v. 2, n. 3, p. 1-15, 2020. DOI: 10.1002/est2.139 Abstract: Pd/C, Au@Pd/C (core-shell), and AuPd/C (AR—consisting in Au microparticles) were used as electrocatalysts for ethanol oxidation in alkaline medium. A synergistic effect between Au and Pd atoms in Au@Pd/C makes the binding between ethanol and Au@Pd/C stronger. This leads to product formation in higher potentials and can be useful to select ethanol products. We also showed that the atomic composition of the electrocatalysts to be used in fuel cells and in powder form to be used in electrochemical experiments are very similar, reaching high values of correlation. The depth profiling X-ray photoelectron spectroscopy for the anode catalysts to be used in fuel cells can provide new insights about ethanol oxidation in direct ethanol fuel cells (DEFCs), for instance, metal oxide species can act in fuel cells environment. In terms of electric generation, Au@Pd/C presented a better performance in electrochemical experiments; the current density was about 1.6 times higher than the peak current density obtained for Pd/C. In terms of electrochemical stability, Au@Pd/C presented better final current density when compared to Pd/C and AuPd/C electrocatalysts. However, in DEFC experiments, Pd/C showed better performance.
Palavras-Chave: electrocatalysts; gold alloys; palladium alloys; direct ethanol fuel cells; x-ray photoelectron spectroscopy; depth; fourier transform spectrometers; attenuation; reflection; in-situ processing
. Comparison of various atomic compositions of Au@Pd/C, Pd/C, and AuPd/C electrocatalysts for direct ethanol fuel cells. Energy Storage, v. 2, n. 3, p. 1-15, 2020. DOI: 10.1002/est2.139. Disponível em: http://repositorio.ipen.br/handle/123456789/31471. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Conversão de metano em metanol com co-geração de energia elétrica a partir de catalisadores de paládio suportados em carbono / Conversion of methane to methanol with co-generation of electricity from palladium catalysts supported in carbon. Revista Virtual de Química, v. 15, n. 2, p. 221-226, 2023. DOI: 10.21577/1984-6835.20220101 Abstract: The application of solid electrolyte reactors for methane oxidation and energy co-generation is attractive, especially with the use of catalysts synthesized from noble metals such as palladium. In this work, we prepared three different compositions of palladium on carbon support to evaluate the composition that had the greatest potential for energy generation. Catalysts in the proportions of 5, 10 and 20% of Pd/C were tested for the conversion of greenhouse gases into organic molecules of higher added value using electrochemical fuel cell solid electrolyte reactors. The focus of this work was the conversion of methane into methanol, using the fuel cell as a reactor and the commercial Pd/C as electrocatalyst. The electrocatalysts were tested at the anode, analyzed by infrared (IR) spectroscopy and their activities verified by experiments with rotating ring disk electrode (RRDE). Higher levels of palladium (Pd/C 20%) favored obtaining electrical power, and the intermediate composition (Pd/C 10%) showed a greater production of less oxidized compounds, such as methanol, in addition to generating electricity.
Palavras-Chave: methane; oxidation; palladium; catalysts; fuel cells
. Conversão de metano em metanol com co-geração de energia elétrica a partir de catalisadores de paládio suportados em carbono. Revista Virtual de Química, v. 15, n. 2, p. 221-226, 2023. DOI: 10.21577/1984-6835.20220101. Disponível em: http://repositorio.ipen.br/handle/123456789/34056. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Desempenho eletrocatalítico de Pd/C e Pt/C para geração de energia a partir do extrato de cana-de-açúcar em célula a combustível de líquido direto / Electrocatalytic performance of Pd/C and Pt/C for power generation from sugarcane extract in direct liquid fuel cell. Revista Virtual de Química, v. 15, n. 2, p. 241-247, 2023. DOI: 10.21577/1984-6835.20220140 Abstract: The processing of biomass to obtain fuels such as ethanol results in generating waste and polluting the environment. However, to meet energy demand and simultaneously reduce environmental pollution, fuel cells are promising devices for converting chemical compounds into electricity. Fuel cells can be powered by various types of liquids, including the sugars available in sugarcane extract, with high energy potential. Fuel cells employ the use of noble metals as electrocatalysts, such as Pt or Pd, to carry out the oxidation of these fuels. In this sense, this work reports the study of the oxidation of sugarcane extract in these different noble metals. The platinum catalyst was shown to be more active for the oxidation of sugars, resulting in a power density 10 times greater than Pd/C using a 50% diluted sugarcane extract solution, resulting in promising fuel cell systems. To produce ecologically correct electrical energy for the industry in general.
Palavras-Chave: biomass; sugar cane; renewable energy sources; electrocatalysts; fuel cells
. Desempenho eletrocatalítico de Pd/C e Pt/C para geração de energia a partir do extrato de cana-de-açúcar em célula a combustível de líquido direto. Revista Virtual de Química, v. 15, n. 2, p. 241-247, 2023. DOI: 10.21577/1984-6835.20220140. Disponível em: http://repositorio.ipen.br/handle/123456789/34055. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Desenvolvimento de novos sistemas de eletrocatalisadores nano-sispersos 20% Pt-(2%Pt-Cesub(0.9)Wsub(0.1)Osub(2))/C tolerantes ao monóxido de carbono (CO) para ânodos de PEMFC / Development of new systems of nano-disperse 20% Pt-(2%Pt-Cesub(0.9)Wsub(0.1)Osub(2))/C tolerant to carbon monoxide (CO) for PEMFC'S anodes . 2011. Dissertação (Mestrado) - Instituto de Pesquisas Energéticas e Nucleares - IPEN-CNEN/SP, São Paulo. 80 p. Orientador: Marcelo Linardi. DOI: 10.11606/D.85.2011.tde-31102011-084235 Abstract: O material (pó) de nanofase de Ce0,9W0,1O2 foi sintetizado por coprecipitação de oxalatos de cério (IV) e cátions de tungstênio (IV). A redução da platina (2%) foi feita pelo método da redução por álcool, utilizando uma solução de ácido hexacloroplatínico (H2PtCl6.6H2O) como fonte do metal, óxido de cério dopado com tungstênio (Ce0,9W0,1O2) utilizado como suporte e, uma solução de etilenoglicol/água (75/25, v/v) como solvente e agente redutor. Os materiais 2%Pt-Ce0,9W0,1O2 foram misturados em Pt/C E-TEK 20%, utilizando-se processo de mistura física para produzir os eletrocatalisadores de 20%Pt-(2%Pt-Ce0,9W0,1O2)/C. Os eletrocatalisadores obtidos foram caracterizados por espectroscopia de energia dispersiva de raios X (EDX) acoplado à microscopia eletrônica de varredura (MEV), análises de difração de raios X (DRX), e microscopia eletrônica de transmissão (MET). O conjunto eletrodos-membrana (MEAs) foram preparados para o ânodo com cargas iguais a 0,401, 0,364, 0,328 mg Pt cm-2 de eletrocatalisadores 20%Pt-(2%Pt-Ce0,9W0,1O2)/C produzidos. No cátodo foi usada uma carga de 0,4 mg Pt cm-2 de eletrocatalisador Pt/C ETEK. A polarização anódica foi realizada para oxidação de H2/CO (100 ppm de CO). A tolerância ao CO foi estudada utilizando o processo eletroquímico (stripping de CO e medidas de curvas de polarização). Os resultados obtidos mostraram que a oxidação de CO adsorvido a CO2 na superfície de platina ocorre em potenciais menos positivos mostrando tolerância ao CO adsorvido nestes eletrocatalisadores (20%Pt-(2%Pt-Ce0,9W0,1O2)/C (50:50, 60:40 e 70:30)) a uma temperatura de 85 ºC e com pressão absoluta de 2 bar para ânodo e cátodo, comparado com Pt/C E-TEK 20%.
Palavras-Chave: electrocatalysts; platinum; cerium; tungsten; cobalt; proton exchange membrane fuel cells; electrochemistry; x-ray spectroscopy; transmission electron microscopy; scanning electron microscopy; x-ray diffraction
. Desenvolvimento de novos sistemas de eletrocatalisadores nano-sispersos 20% Pt-(2%Pt-Cesub(0.9)Wsub(0.1)Osub(2))/C tolerantes ao monóxido de carbono (CO) para ânodos de PEMFC. Orientador: Marcelo Linardi. 2011. 80 f. Dissertação (Mestrado) - Instituto de Pesquisas Energéticas e Nucleares - IPEN-CNEN/SP, São Paulo. DOI: 10.11606/D.85.2011.tde-31102011-084235. Disponível em: http://repositorio.ipen.br/handle/123456789/10029. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. O efeito da concentração de etanol na performance e na distribuição de produtos em uma DEFC: um estudo utilizando o acessório DEFC/ATR-FTIR. In: Congreso de la Sociedad Iberoamericana de Electroquimica, 21st, 6-11 de abril, 2014, La Serena, Chile. 2014.
Palavras-Chave: direct ethanol fuel cells; electrocatalysts; platinum; tin; carbon; acetic acid; fourier transform spectrometers
. O efeito da concentração de etanol na performance e na distribuição de produtos em uma DEFC: um estudo utilizando o acessório DEFC/ATR-FTIR. In: Congreso de la Sociedad Iberoamericana de Electroquimica, 21st, 6-11 de abril, 2014, La Serena, Chile. 2014. Disponível em: http://repositorio.ipen.br/handle/123456789/23203. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Effect of TiO2 and synthesis strategies on formate oxidation: electrochemical and fuel cell approaches. Electrocatalysis, v. 14, n. 2, p. 221-231, 2023. DOI: 10.1007/s12678-022-00789-5 Abstract: Direct formate fuel cells have gained increasing attention since formate can be obtained by CO2 reduction, being shown as a renewable power source. This paper reports the use of Pd nanoparticles supported on physical mixtures of Vulcan carbon and TiO2 in different ratios and different Pd reduction methodologies. The materials were prepared using sodium borohydride as a reducing agent and analyzed toward formate oxidation in alkaline media. The prepared electrocatalysts showed peaks of Pd face-centered cubic and TiO2 anatase and rutile phases and an average particle size between 3.7 and 7.9 nm. Experiments considering formate electro-oxidation (voltammetry and chronoamperometry) showed that the presence of TiO2 is favorably using both synthesis methodologies while single cells revealed Pd nanoparticles supported on physical mixtures of carbon and TiO2, in the proportion of 75:25 as the most efficient, which was explained by the carbon high electrical conductivity and small quantities of TiO2 working as co-catalyst.
Palavras-Chave: palladium; nanoparticles; titanium oxides; oxidation; formate fuel cells
. Effect of TiO2 and synthesis strategies on formate oxidation: electrochemical and fuel cell approaches. Electrocatalysis, v. 14, n. 2, p. 221-231, 2023. DOI: 10.1007/s12678-022-00789-5. Disponível em: http://repositorio.ipen.br/handle/123456789/33980. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Effects of TiO2 in Pd-TiO2/C for glycerol oxidation in a direct alkaline fuel cell. Journal of Fuel Chemistry and Technology, v. 50, n. 4, p. 474-483, 2022. DOI: 10.1016/S1872-5813(21)60171-8 Abstract: The Pd-TiO2 electrocatalysts were synthesized via sodium borohydride reduction and characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), cyclic voltammetry, chronoamperometry and attenuated total reflectance-Fourier transform infrared (ATR-FTIR). The X-ray diffraction experiments of the Pd-TiO2 showed peaks associated with Pd face-centered cubic (fcc) structure and peaks characteristics of TiO2 (anatase phase) with a tetragonal structure. The TEM images showed that the Pd and TiO2 nanoparticles were well distributed in the carbon support showing some clustered regions with nanoparticle sizes between 7 and 8 nm. Cyclic voltammograms showed an increase in current density values after the glycerol adsorption process. Experiments in alkaline direct glycerol fuel cells at 60 °C showed a higher power density for Pd-TiO2/C (70:30) in comparison to the commercial Pd/C electrocatalyst indicating that the use of the TiO2 co-catalyst with Pd nanoparticles had a beneficial behavior. This effect can be attributed to the electronic effect or to the bifunctional mechanism. Molecules with high-value added glyceraldehyde, hydroxypyruvate and formate were identified as electrochemical reaction products of glycerol on all prepared electrocatalysts.
Palavras-Chave: electrocatalysts; titanium; palladium; glycerol; alkaline electrolyte fuel cells; fuel cells; electrocatalysts; voltametry
. Effects of TiO2 in Pd-TiO2/C for glycerol oxidation in a direct alkaline fuel cell. Journal of Fuel Chemistry and Technology, v. 50, n. 4, p. 474-483, 2022. DOI: 10.1016/S1872-5813(21)60171-8. Disponível em: http://repositorio.ipen.br/handle/123456789/33469. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Electrocatalytic oxidation of methane in an acidic electrolyte using PdMn/C-ITO electrocatalysts synthetized by sodium borohydride reduction process. In: ENERGY TRANSITION RESEARCH AND INNOVATION, October 1-2, 2019, São Paulo, SP. Abstract... São Paulo: Research Centre for Gas Innovation, 2019. Abstract: Pd/C-ITO and PdMn/C-ITO electrocatalysts with different atomic ratio (90:10, 70:30 and 50:50) were synthetized by borohydride reduction method and characterized by X-ray diffraction, Transmission electronic microscopy and electrochemical studies (cyclic voltammetry). The electrochemical studies showed that PdMn(50:50)/C-ITO had superior performance for electrochemical oxidation of methane in an acidic electrolyte at 25 oC compared to others electrocatalysts prepared. The experiments in a single DMEFC also showed that the PdMn(70:30)/C-ITO electrocatalyst exhibited higher performance for methane oxidation in comparison with Pd/C-ITO, PdMn(90:10)/C-ITO and PdMn(50:50)/C-ITO electrocatalysts. These result indicated that the addition of Mn to Pd favor the electrochemical oxidation of Methane in acid medium, where this effect could be attributed to the synergy between the constituents of the binary electrocatalysts or to electronic modification of Pd atoms by the neighboring Mn atoms as the proximity of Pd and Mn atoms on the surface of the C-ITO (bifunctional mechanism - the presence of Pd and Mn oxides species).. Electrocatalytic oxidation of methane in an acidic electrolyte using PdMn/C-ITO electrocatalysts synthetized by sodium borohydride reduction process. In: ENERGY TRANSITION RESEARCH AND INNOVATION, October 1-2, 2019, São Paulo, SP. Abstract... São Paulo: Research Centre for Gas Innovation, 2019. Disponível em: http://repositorio.ipen.br/handle/123456789/30865. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Methane activation at low temperature in an acidic electrolyte using PdAu/C, PdCu/C, and PdTiO2/C electrocatalysts for PEMFC. Research on Chemical Intermediates, v. 46, n. 5, p. 2481-2496, 2020. DOI: 10.1007/s11164-020-04102-1 Abstract: Pd/C, PdAu/C, PdCu/C, and PdTiO2/ C electrocatalysts were prepared by a sodium borohydride reduction process for methane activation at low temperatures in a PEMFC reactor. These electrocatalysts were characterized by XRD, TEM, XPS, ICP-MS, ATR-FTIR, and cyclic voltammetry. The diffractograms of Pd/C, PdAu(50:50)/C, PdCu(50:50)/C, and PdTiO2( 50:50)/C electrocatalysts showed peaks associated with Pd face-centered cubic structure. PdAu(50:50)/C showed a small shift in the peak center when it was compared to Pd/C, while PdCu(50:50)/C showed a shift to higher angles when it was also compared to Pd/C. This effect can be due to the formation of an alloy between Pd and Au, and Pd and Cu. By TEM experiments, a mean nanoparticle size was observed between 6.9 and 8.9 nm for all electrocatalysts. Cyclic voltammograms of Pd/C, PdAu/C, PdCu/C and PdTiO2/ C electrocatalysts showed an increase in current density values after the adsorption of methane The ATR-FTIR experiments showed for all electrocatalysts the formation of methanol and formic acidic. Polarization curves at 80 °C acquired in a PEMFC reactor showed that PdAu(50:50)/C and PdTiO2( 50:50)/C had superior performance when compared to Pd/C, indicating the beneficial effect of adding the co-catalyst; this behavior has been attributed to the bifunctional mechanism or electronic effect.
Palavras-Chave: methane; electrocatalysts; borohydrides; sodium compounds; binary alloy systems; palladium alloys; gold alloys; copper alloys; titanium oxides; proton exchange membrane fuel cells; temperature range 0065-0273 k; fourier transformation; infrared spectra; attenuation; reflection
. Methane activation at low temperature in an acidic electrolyte using PdAu/C, PdCu/C, and PdTiO2/C electrocatalysts for PEMFC. Research on Chemical Intermediates, v. 46, n. 5, p. 2481-2496, 2020. DOI: 10.1007/s11164-020-04102-1. Disponível em: http://repositorio.ipen.br/handle/123456789/31446. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Methane to methanol conversion using proton-exchange membrane fuel cells and PdAu/antimony-doped tin oxide nanomaterials. Methane, v. 2, n. 3, p. 252–264, 2023. DOI: 10.3390/methane2030017 Abstract: This study investigates the use of Au-doped Pd anodic electrocatalysts on ATO support for the conversion of methane to methanol. The study uses cyclic voltammetry, in situ Raman spectra, polarization curves, and FTIR analysis to determine the optimal composition of gold and palladium for enhancing the conversion process. The results demonstrate the potential for utilizing methane as a feedstock for producing sustainable energy sources. The Pd75Au25/ATO electrode exhibited the highest OCP value, and Pd50Au50/ATO had the highest methanol production value at a potential of 0.05 V. Therefore, it can be concluded that an optimal composition of gold and palladium exists to enhance the conversion of methane to methanol. The findings contribute to the development of efficient and sustainable energy sources, highlighting the importance of exploring alternative ways to produce methanol.. Methane to methanol conversion using proton-exchange membrane fuel cells and PdAu/antimony-doped tin oxide nanomaterials. Methane, v. 2, n. 3, p. 252–264, 2023. DOI: 10.3390/methane2030017. Disponível em: http://repositorio.ipen.br/handle/123456789/34366. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Niobium as co-electrocatalyst for Pd in alkaline medium: improved selectivity and mechanism for ethanol electrooxidation reaction. In: REUNIAO ANUAL DA SOCIEDADE BRASILEIRA DE QUIMICA: CONSTRUINDO O AMANHA, 41., 21-24 de maio, 2018, Foz do Iguaçu, PR. Resumo... São Paulo: Sociedade Brasileira de Química, 2018. Abstract: Direct ethanol fuel cells (DEFC) have been evaluated as a possible alternative source of sustainable energy, because it can reach high current densities with minimal pollutant production [1]. In this work, we report the synthesis of PdxNby/C binary electrocatalysts supported on Vulcan XC-72 carbon by the sol-gel method [2]. These synthesized electrocatalysts were physically characterized by DRX, EDS, ICP-MS, XPS and TEM. Their electrochemical activities (CV and CA) were evaluated as already reported by Souza [2]. Here, we reported new results from Tafel plots and FTIR ex situ experiments for ethanol oxidation reaction using those electrocatalysts. Pd/C electrocatalyst has the EOR governed by two determining steps since it showed two straight lines between 570 mV and 730mV, presenting also two different slopes (a and b, in Figure 1). Unlike the other electrocatalysts, with Nb in their chemical composition, that the EOR occurs by one determining step since there is just one slope. The exchange current densities (A cm-2) were 2.3x10-17, 2.7x10-12, 6.6x10-10, 3.6x10-11 and 7.3x10-11 for Pd/Ca, Pd/Cb, Pd1Nb1/C, Pd3Nb1/C and Pd1Nb3/C, respectively. This shows that Nb increases the electron exchange rate at the analyte/electrode interface, improving the kinetics of the EOR reaction [3]. FTIR experiments strengthened the evidence that Nb modifies the Pd mechanism for EOR electrocatalysis to a mechanism that present almost no formation of acetaldehyde, avoiding the reaction ... The FTIR spectra showed that Pd1Nb1/C displays the highest production of CO2 and the lowest production of acetaldehyde. Furthermore, the ADT experiments with ICP-MS analysis indicated that Pd1Nb1/C obtained the highest peak current density during 1000 cycles of the experiment, presenting the lowest Pd mass loss after the ADT.. Niobium as co-electrocatalyst for Pd in alkaline medium: improved selectivity and mechanism for ethanol electrooxidation reaction. In: REUNIAO ANUAL DA SOCIEDADE BRASILEIRA DE QUIMICA: CONSTRUINDO O AMANHA, 41., 21-24 de maio, 2018, Foz do Iguaçu, PR. Resumo... São Paulo: Sociedade Brasileira de Química, 2018. Disponível em: http://repositorio.ipen.br/handle/123456789/29720. Acesso em: $DATA.Como referenciar este item
Esta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Pd, Ag and Bi carbon-supported electrocatalysts as electrochemical multifunctional materials for ethanol oxidation and dopamine determination. Electrochimica Acta, v. 428, p. 1-13, 2022. DOI: 10.1016/j.electacta.2022.140932 Abstract: This manuscript describes the investigation towards the multifunctional synthesis, characterization, and application of different Pd, Ag and Bi-carbon black supported electrocatalysts in two different fields in electrochemistry: fuel cells and electrochemical sensors. Throughout morphological and electrochemical characterizations, comprising scanning and transmission electron microscopies, X-ray powder diffraction, electrochemical impedance spectroscopy, and cyclic voltammetry techniques, the materials were characterized to better understand their properties towards proposed applications. Afterward, the materials were employed for ethanol oxidation in alkaline media, with investigations by chronoamperometry, cyclic voltammetry, and by closing a direct alkaline fuel cell, which the Pd50Ag45Bi05/C composite presented attractive ethanol catalysis behavior, with a maximum power density of 19.70 mW cm−2, at 30.59 mA cm−2. Also, the proposed device was applied for dopamine determination by square wave voltammetry. In this sense, two linear behaviors, respectively ranging from 0.2 to 1.0 and 4.0 to 40 μmol L−1 were obtained, due to two distinctive mechanisms. This higher activity has been attributed to the synergism among the used metals and proportions contributing to the bifunctional and electronic effects. As synthetic samples investigations were accomplished, data reinforces the proposed material as a possible interfacing composite in electrochemistry.
Palavras-Chave: electrocatalysts; ethanol; oxidation; dopamine
. Pd, Ag and Bi carbon-supported electrocatalysts as electrochemical multifunctional materials for ethanol oxidation and dopamine determination. Electrochimica Acta, v. 428, p. 1-13, 2022. DOI: 10.1016/j.electacta.2022.140932. Disponível em: http://repositorio.ipen.br/handle/123456789/33413. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Structural analysis of PdRh/C and PdSn/C and its use as electrocatalysts for ethanol oxidation in alkaline medium. International Journal of Hydrogen Energy, v. 44, n. 2, p. 937-951, 2019. DOI: 10.1016/j.ijhydene.2018.11.049 Abstract: The Pd/C, PdRh(50:50)/C and PdSn(50:50)/C nanomaterials were used as electrocatalysts for ethanol (EtOH) oxidation in Direct Ethanol Fuel Cell (DEFC) in an alkaline medium. This work aims to provide a complete physical characterization of the nanomaterials, elucidate the bifunctional mechanism concerning ethanol oxidation reaction and understand the influence of carbon e metal bonding in the electrochemical activity. These nanomaterials were investigated by X-ray photoelectron spectroscopy (XPS) and revealed that the atomic percentage of the surface is different of those obtained by Energy Dispersive X-ray spectroscopy (EDS). Raman spectroscopy showed a bonding between palladium and carbon atoms which can play a decisive role in the performance of the materials. Attenuated Total Reflectance technique coupled to the Fourier Transform Infrared spectroscopy (ATR-FTIR) made possible to investigate the oxidation products originated by the ethanol oxidation, and all the electrocatalysts showed the presence of acetaldehyde, carbonate ions, acetate and carbon dioxide, suggesting that the mechanism of oxidation is incomplete. Among all the nanomaterials studied, PdSn(50:50)/C showed the best electrochemical and Fuel Cell's results. It is about 33% better than Pd/C. The micrographs obtained by Transmission Electron Microscopy (TEM) revealed some agglomerate regions, but they are consistent with the literature data.
Palavras-Chave: electrocatalysts; ethanol; x-ray diffraction; direct ethanol fuel cells; oxidation; nanomaterials; raman spectroscopy
. Structural analysis of PdRh/C and PdSn/C and its use as electrocatalysts for ethanol oxidation in alkaline medium. International Journal of Hydrogen Energy, v. 44, n. 2, p. 937-951, 2019. DOI: 10.1016/j.ijhydene.2018.11.049. Disponível em: http://repositorio.ipen.br/handle/123456789/30033. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. The effect of support on Pd1Nb1 electrocatalysts for ethanol fuel cells. Renewable Energy, v. 150, p. 293-306, 2020. DOI: 10.1016/j.renene.2019.12.110 Abstract: Pd1Nb1/C on different kinds of carbon black were prepared by a modified sol-gel method. The alkaline direct ethanol fuel cell (ADEFC) performance was performed first with the Pd1Nb1 electrocatalysts and then by varying the fuel concentration. In CV, Pd1Nb1/Printex 6L (50:50 wt%) exhibited 2.2 times higher mass activity than that of the Pd/C (Alfa Aesar); their mass activities were 1300 and 590 mA mg 1 Pd , respectively. The best performance for the ADEFC was obtained using Pd1Nb1/Printex 6L, which yielded a maximum power density and cell voltage of 28 mW cm 2 and 1.17 V, respectively. The Pd1Nb1/Printex 6L electrocatalyst exhibited a more negative onset potential for the CO stripping reaction. We suggest that the higher hydrophilicity (contact angle) and higher degree of disorder of Printex 6L (Raman) corroborates these results. In addition, both bifunctional and electronic effects operated on the electrocatalyst due to the presence of metal oxides and alloys of PdNb (XRD), respectively, in the synthesized electrocatalysts. Therefore, it was notable that the support has an essential roledas important as the cocatalystdin the electrocatalytic performance.
Palavras-Chave: ethanol fuels; direct ethanol fuel cells; ethanol; oxidation; palladium; niobium; sol-gel process; electrochemistry; electrocatalysts
. The effect of support on Pd1Nb1 electrocatalysts for ethanol fuel cells. Renewable Energy, v. 150, p. 293-306, 2020. DOI: 10.1016/j.renene.2019.12.110. Disponível em: http://repositorio.ipen.br/handle/123456789/31445. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
Itens para a visualização no momento 1-18 de 18
Buscar no repositório
Navegar
Minha conta
Visualizar
A pesquisa no RD utiliza os recursos de busca da maioria das bases de dados. No entanto algumas dicas podem auxiliar para obter um resultado mais pertinente.
✔ É possível efetuar a busca de um autor ou um termo em todo o RD, por meio do Buscar no Repositório , isto é, o termo solicitado será localizado em qualquer campo do RD. No entanto esse tipo de pesquisa não é recomendada a não ser que se deseje um resultado amplo e generalizado.
✔ A pesquisa apresentará melhor resultado selecionando um dos filtros disponíveis em Navegar
✔ Os filtros disponíveis em Navegar tais como: Coleções, Ano de publicação, Títulos, Assuntos, Autores, Revista, Tipo de publicação são autoexplicativos. O filtro, Autores IPEN apresenta uma relação com os autores vinculados ao IPEN; o ID Autor IPEN diz respeito ao número único de identificação de cada autor constante no RD e sob o qual estão agrupados todos os seus trabalhos independente das variáveis do seu nome; Tipo de acesso diz respeito à acessibilidade do documento, isto é , sujeito as leis de direitos autorais, ID RT apresenta a relação dos relatórios técnicos, restritos para consulta das comunidades indicadas.

A opção Busca avançada utiliza os conectores da lógica boleana, é o melhor recurso para combinar chaves de busca e obter documentos relevantes à sua pesquisa, utilize os filtros apresentados na caixa de seleção para refinar o resultado de busca. Pode-se adicionar vários filtros a uma mesma busca.
Exemplo:
Buscar os artigos apresentados em um evento internacional de 2015, sobre loss of coolant, do autor Maprelian.
Autor: Maprelian
Título: loss of coolant
Tipo de publicação: Texto completo de evento
Ano de publicação: 2015
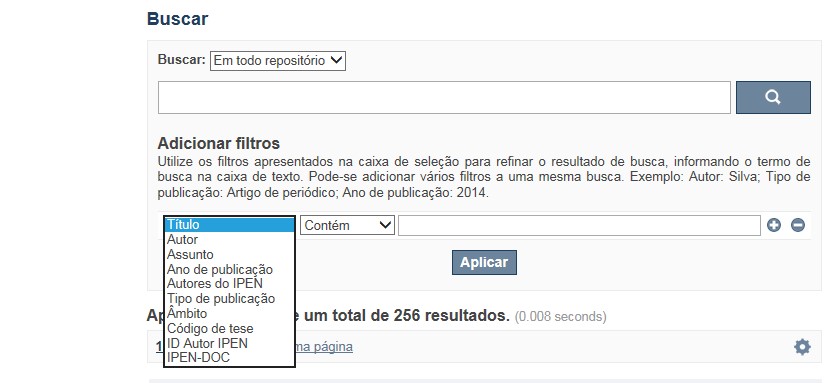
✔ Para indexação dos documentos é utilizado o Thesaurus do INIS, especializado na área nuclear e utilizado em todos os países membros da International Atomic Energy Agency – IAEA , por esse motivo, utilize os termos de busca de assunto em inglês; isto não exclui a busca livre por palavras, apenas o resultado pode não ser tão relevante ou pertinente.
✔ 95% do RD apresenta o texto completo do documento com livre acesso, para aqueles que apresentam o ![]() significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
✔ Ao efetuar a busca por um autor o RD apresentará uma relação de todos os trabalhos depositados no RD. No lado direito da tela são apresentados os coautores com o número de trabalhos produzidos em conjunto bem como os assuntos abordados e os respectivos anos de publicação agrupados.
✔ O RD disponibiliza um quadro estatístico de produtividade, onde é possível visualizar o número dos trabalhos agrupados por tipo de coleção, a medida que estão sendo depositados no RD.
✔ Na página inicial nas referências são sinalizados todos os autores IPEN, ao clicar nesse símbolo ![]() será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
✔ Na página do pesquisador, é possível verificar, as variações do nome, a relação de todos os trabalhos com texto completo bem como um quadro resumo numérico; há links para o Currículo Lattes e o Google Acadêmico ( quando esse for informado).
ATENÇÃO!
ESTE TEXTO "AJUDA" ESTÁ SUJEITO A ATUALIZAÇÕES CONSTANTES, A MEDIDA QUE NOVAS FUNCIONALIDADES E RECURSOS DE BUSCA FOREM SENDO DESENVOLVIDOS PELAS EQUIPES DA BIBLIOTECA E DA INFORMÁTICA.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
1. Portaria IPEN-CNEN/SP nº 387, que estabeleceu os princípios que nortearam a criação do RDI, clique aqui.
2. A experiência do Instituto de Pesquisas Energéticas e Nucleares (IPEN-CNEN/SP) na criação de um Repositório Digital Institucional – RDI, clique aqui.
O Repositório Digital do IPEN é um equipamento institucional de acesso aberto, criado com o objetivo de reunir, preservar, disponibilizar e conferir maior visibilidade à Produção Científica publicada pelo Instituto, desde sua criação em 1956.
Operando, inicialmente como uma base de dados referencial o Repositório foi disponibilizado na atual plataforma, em junho de 2015. No Repositório está disponível o acesso ao conteúdo digital de artigos de periódicos, eventos, nacionais e internacionais, livros, capítulos, dissertações, teses e relatórios técnicos.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
