Navegação IPEN por assunto "drug delivery"
- Página inicial
- →
- IPEN
- →
- Navegação IPEN por assunto
- Sobre
- Perfil Técnico
- Política de funcionamento
- Ajuda
- Apresentação
Navegação IPEN por assunto "drug delivery"
Itens para a visualização no momento 1-20 de 24
-
. About the sterilization of chitosan hydrogel nanoparticles. Plos One, v. 11, n. 12, 2016. DOI: 10.1371/journal.pone.0168862 Abstract: In the last years, nanostructured biomaterials have raised a great interest as platforms for delivery of drugs, genes, imaging agents and for tissue engineering applications. In particular, hydrogel nanoparticles (HNP) associate the distinctive features of hydrogels (high water uptake capacity, biocompatibility) with the advantages of being possible to tailor its physicochemical properties at nano-scale to increase solubility, immunocompatibility and cellular uptake. In order to be safe, HNP for biomedical applications, such as injectable or ophthalmic formulations, must be sterile. Literature is very scarce with respect to sterilization effects on nanostructured systems, and even more in what concerns HNP. This work aims to evaluate the effect and effectiveness of different sterilization methods on chitosan (CS) hydrogel nanoparticles. In addition to conventional methods (steam autoclave and gamma irradiation), a recent ozone-based method of sterilization was also tested. A model chitosan-tripolyphosphate (TPP) hydrogel nanoparticles (CS-HNP), with a broad spectrum of possible applications was produced and sterilized in the absence and in the presence of protective sugars (glucose and mannitol). Properties like size, zeta potential, absorbance, morphology, chemical structure and cytotoxicity were evaluated. It was found that the CS-HNP degrade by autoclaving and that sugars have no protective effect. Concerning gamma irradiation, the formation of agglomerates was observed, compromising the suspension stability. However, the nanoparticles resistance increases considerably in the presence of the sugars. Ozone sterilization did not lead to significant physical adverse effects, however, slight toxicity signs were observed, contrarily to gamma irradiation where no detectable changes on cells were found. Ozonation in the presence of sugars avoided cytotoxicity. Nevertheless, some chemical alterations were observed in the nanoparticles.
Palavras-Chave: sterilization; oligosaccharides; hydrogels; nanoparticles; drug delivery; biological materials
. About the sterilization of chitosan hydrogel nanoparticles. Plos One, v. 11, n. 12, 2016. DOI: 10.1371/journal.pone.0168862. Disponível em: http://repositorio.ipen.br/handle/123456789/27898. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. An abraded surface of doxorubicin-loaded surfactant-containing drug delivery systems effectively reduces the survival of carcinoma cells. Biomedicines, v. 4, n. 3, 2016. DOI: 10.3390/biomedicines4030022 Abstract: An effective antitumor remedy is yet to be developed. All previous approaches for a targeted delivery of anticancer medicine have relied on trial and error. The goal of this study was to use structural insights gained from the study of delivery systems and malignant cells to provide for a systematic approach to the development of next-generation drugs. We used doxorubicin (Dox) liposomal formulations. We assayed for cytotoxicity via the electrical current exclusion method. Dialysis of the samples yielded information about their drug release profiles. Information about the surface of the delivery systems was obtained through synchrotron small-angle X-ray scattering (SAXS) measurements. SAXS measurements revealed that Dox-loading yielded an abraded surface of our Dox liposomal formulation containing soybean oil, which also correlated with an effective reduction of the survival of carcinoma cells. Furthermore, a dialysis assay revealed that a higher burst of Dox was released from soybean oil-containing preparations within the first five hours. We conclude from our results that an abraded surface of Dox-loaded drug delivery system increases their efficacy. The apparent match between surface geometry of drug delivery systems and target cells is suggested as a steppingstone for refined development of drug delivery systems. This is the first study to provide a systematic approach to developing next-generation drug carrier systems using structural insights to guide the development of next-generation drug delivery systems with increased efficacy and reduced side effects.
Palavras-Chave: drug delivery; doxorubicin; neoplasms; therapy; synchrotron radiation; small angle scattering; x-ray diffraction; in vitro; carcinomas
. An abraded surface of doxorubicin-loaded surfactant-containing drug delivery systems effectively reduces the survival of carcinoma cells. Biomedicines, v. 4, n. 3, 2016. DOI: 10.3390/biomedicines4030022. Disponível em: http://repositorio.ipen.br/handle/123456789/27834. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Chemical and structural characterization of hybrid delivery systems studied by FTIR, NMR, and SAS techniques. In: KESHARWANI, P. (Ed.); JAIN, N.K. (Ed.). Hybrid Nanomaterials for Drug Delivery. Cambridge, MA, United States: Woodhead Publishing, 2022. p. 27-51, cap. 2. (Woodhead Publishing Series in Biomaterials). DOI: 10.1016/B978-0-323-85754-3.00005-8 Abstract: In recent years, the research for new classes of materials for biomedical applications has directed the synthesis and design of hybrid systems composed of two or more components (metals, clays, ceramics, bioglass, polymers, lipids, enzymes) conferring to them multifunctional properties, in general synergistic, different to those obtained for their parent components. One of the main advantages attributed to hybrid systems is their ability to modulate the drug release rate into the biological medium, which improve their therapeutic efficacy. However, this enhanced biomedical performance is a result of chemical interactions among material components that assume different levels of structural organization. This chapter provides a discussion about applications of three physicochemical techniques used for characterizing those materials, Fourier-transform infrared spectroscopy, nuclear magnetic resonance, and small-angle scattering. Therefore a brief introduction on fundamental techniques followed by a discussion on relationships between composition and main chemical and structural finding are covered.
Palavras-Chave: nanomaterials; medical supplies; drug delivery; hybrid systems
. Chemical and structural characterization of hybrid delivery systems studied by FTIR, NMR, and SAS techniques. In: KESHARWANI, P. (ed.); JAIN, N.K. (ed.). Hybrid Nanomaterials for Drug Delivery. Cambridge, MA, United States: Woodhead Publishing, 2022. cap. 2. p. 27-51. (Woodhead Publishing Series in Biomaterials). DOI: 10.1016/B978-0-323-85754-3.00005-8. Disponível em: http://repositorio.ipen.br/handle/123456789/33235. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Complexation of oxethazaine with 2-hydroxypropyl-bcyclodextrin: increased drug solubility, decreased cytotoxicity and analgesia at inflamed tissues. Journal of Pharmacy and Pharmacology, v. 69, n. 6, p. 652-662, 2017. DOI: 10.1111/jphp.12703 Abstract: ObjectivesOxethazaine (OXZ) is one of the few local anaesthetics that provides analgesia at low pH, but presents poor solubility, cytotoxicity and no parenteral formulations. To address these issues, we aimed to prepare OXZ host-guest inclusion complex with hydroxypropyl-beta-cyclodextrin (HP--CD). MethodsThe inclusion complex was formed by co-solubilization, followed by a job plot analysis to determine stoichiometry of complexation and dialysis equilibrium analysis (based on UV/VIS absorption and fluorescence profiles of OXZ). Complex formation was confirmed by phase-solubility data, X-ray, Scanning Electron Microscopy and DOSY-H-1-NMR experiments. In vitro cytotoxicity was analysed by MTT test in 3T3 fibroblasts. In vivo analgesia was tested by Von Frey test (inflammatory wounds - rats). Key findingsOxethazaine complexed (1 : 1 molar ratio) with HP--CD, as indicated by loss of OZX crystalline structure (X-ray) and strong host: guest interaction (NMR, K = 198/M), besides increased solubility. In vitro cell survival improved with the complex (IC50 OXZ = 28.9 m, OXZ : HP--CD = 57.8 m). In addition, the complex (0.1% OXZ) promoted in vivo analgesia for the same time that 2% lidocaine/epinephrine did. Conclusion Our results show that complexation improved physicochemical and biological properties of OXZ, allowing its application to inflamed tissues by parenteral routes.
Palavras-Chave: analgesics; anesthesia; bioassay; drug delivery; pharmacology; drugs; therapeutic doses
. Complexation of oxethazaine with 2-hydroxypropyl-bcyclodextrin: increased drug solubility, decreased cytotoxicity and analgesia at inflamed tissues. Journal of Pharmacy and Pharmacology, v. 69, n. 6, p. 652-662, 2017. DOI: 10.1111/jphp.12703. Disponível em: http://repositorio.ipen.br/handle/123456789/27866. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Effects of doxorubicin on the structural and morphological characterization of solid lipid nanoparticles (SLN) using small angle neutron scattering (SANS) and small angle X-ray scattering (SAXS). Physica B: Condensed Matter, v. 551, p. 191-196, 2018. DOI: 10.1016/j.physb.2017.12.036 Abstract: Cancer is still a major public health problem. Leaving detection of early stages of tumors and other issues aside, minimizing unwarranted side effects, for example after clinical usage of a liposomal anticancer drug doxorubicin (Dox) formulation, is an unmet clinical problem and the focus of many studies. Using compounds typically used in the preparation of food and/or cosmetics to prepare drug carrier systems, we observed that sodium tetradecyl sulfate (STS) containing and soybean-oil based carriers are more efficient in reducing the viability of HeLa cells tumor cells in comparison to their respective counterparts. Here, we probe the doxorubicin (Dox) loading on structural properties of either soybean oil or coconut oil (Mygliol 812) formulations. Small angle neutron scattering (SANS) assays were performed using V16 instrument at Helmholtz-Zentrum Berlin (HZB) at 25, 37 and 40 C with two or 11m distance between detector and sample to assess a wide range in scattering vector Q. Combined with previous measurement using small angle X-ray scattering (SAXS), our results show that the Dox has different influence in the surface structure of the solid lipid nanoparticles (SLN) and also affects the fractality in the vesicle aggregates when the concentration of the drug is altered, for Mygliol and soybean oil SLN drug delivery carrier systems.
Palavras-Chave: doxorubicin; drug delivery; hela cells; nanoparticles; neoplasms; neutron diffraction; small angle scattering; sodium sulfates; solids; soybean oil; soybean oil; x-ray diffraction
. Effects of doxorubicin on the structural and morphological characterization of solid lipid nanoparticles (SLN) using small angle neutron scattering (SANS) and small angle X-ray scattering (SAXS). Physica B: Condensed Matter, v. 551, p. 191-196, 2018. DOI: 10.1016/j.physb.2017.12.036. Disponível em: http://repositorio.ipen.br/handle/123456789/28506. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Encapsulation study of citalopram and risperidone into nanostructured silica SBA-15 for in vitro release evaluation. Journal of Thermal Analysis and Calorimetry, v. 127, n. 2, p. 1725-1732, 2017. DOI: 10.1007/s10973-016-5995-4 Abstract: Ordered mesoporous silica, SBA-15, presents non-toxic nature; high pore volume and high surface area; thermal, hydrothermal and mechanical stabilities. These features may enable its use as a carrier for commonly prescribed drugs in psychiatric practice: citalopram (CIT) and risperidone (RIS). Thermogravimetry/derivative thermogravimetry (TG/DTG) and ultraviolet and visible absorption spectrophotometry (UV-Vis) were used to determine drug quantity into/onto ECIT (CIT encapsulated into SBA-15), ERIS (RIS encapsulated into SBA-15), MCIT (mixture of CIT with SBA-15) and MRIS (mixture of RIS with SBA-15). All materials were characterized through: TG/DTG, differential scanning calorimetry, elemental analysis, Fourier transform infrared absorption spectrophotometry and nitrogen adsorption-desorption. A general solvent-based method for loading these psychoactive drugs into non-functionalized SBA-15 showed to retard their liberation in simulated gastric and intestinal media when compared to physical mixtures performance. It is expected that the functionalization of this nanostructured silica may increasingly extend the dissolution of CIT and RIS after encapsulation process, leading to application in controlled release systems.
Palavras-Chave: silica; drug delivery; encapsulation; solvent extraction; organic solvents; loading
. Encapsulation study of citalopram and risperidone into nanostructured silica SBA-15 for in vitro release evaluation. Journal of Thermal Analysis and Calorimetry, v. 127, n. 2, p. 1725-1732, 2017. DOI: 10.1007/s10973-016-5995-4. Disponível em: http://repositorio.ipen.br/handle/123456789/27870. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Evaluation of structural changes of benzocaine-loaded, optimized nanostructured lipid carriers using SANS and Raman imaging approaches. In: CONGRESS OF THE INTERNATIONAL UNION FOR PURE APPLIED BIOPHYSICS, 20th; ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 50th; CONGRESS OF BRAZILIAN BIOPHYSICS SOCIETY, 45th; BRAZILIAN SOCIETY ON NUCLEAR BIOSCIENCES CONGRESS, 13th, October 4-8, 2021, São Paulo, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular (SBBq), 2021. p. 183-183. Abstract: Local anesthetics are substances that reversibly block the nerve-impulse conduction, alleviating pain without loss of consciousness. Benzocaine, a poorly soluble local anesthetic, is an ester of para-aminobenzoic acid. Several strategies of formulations can be used to improve bioavailability and decrease adverse effects of benzocaine. In this study nanostructured lipid carriers (NLC) were employed. These lipid-based drug delivery carriers have a lipid core composed of a blend of solid and liquid lipids, and a shelf of non-ionic surfactant. The main aim of this work was to optimize benzocaine-loaded NLC and to investigate structural changes in these nanoparticles, under different temperatures. The ratio of excipients (cetyl palmitate, Capmul® PG-8 NF and Pluronic®F68) and benzocaine in the NLC was optimized using a 2 3 factorial design with respect to the following parameters: particle size, polydispersity index (PDI) and zeta potentials. The interactions between the factors were found relevant to determine particle size and PDI. Using desirability function, the best formulation conditions were found. Structural changes in optimized NLC were observed with Small-Angle Neutron Scattering (SANS) and Raman imaging, in samples at 27, 37 and 40º C. SANS pointed the formation of lamellar structures inside the NLC, which interlamellar distances increase at higher temperature. Raman imaging showed that the incorporation of P68 and benzocaine in-between the lipids increased at higher temperatures, explaining the changes in Q values (SANS). This work shows how different scattering techniques can provide complementary information and be used together to characterize and understand the physical, chemical, and structural changes on the organization of pharmaceutical carriers in drug delivery system.
Palavras-Chave: drugs; pharmacology; anesthetics; drug delivery; design; raman spectra
. Evaluation of structural changes of benzocaine-loaded, optimized nanostructured lipid carriers using SANS and Raman imaging approaches. In: CONGRESS OF THE INTERNATIONAL UNION FOR PURE APPLIED BIOPHYSICS, 20th; ANNUAL MEETING OF THE BRAZILIAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY, 50th; CONGRESS OF BRAZILIAN BIOPHYSICS SOCIETY, 45th; BRAZILIAN SOCIETY ON NUCLEAR BIOSCIENCES CONGRESS, 13th, October 4-8, 2021, São Paulo, SP. Abstract... São Paulo, SP: Sociedade Brasileira de Bioquímica e Biologia Molecular (SBBq), 2021. p. 183-183. Disponível em: http://repositorio.ipen.br/handle/123456789/32868. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. International Journal of Biological Macromolecules, v. 111, p. 1245-1254, 2018. DOI: 10.1016/j.ijbiomac.2018.01.064 Abstract: In this study,we reported the hyaluronic acid (HA) on supramolecular structure of Pluronic F-127 (PLF-127) and/ or Pluronic F-108 (PLF-127) hydrogels, as well as their effects on release mechanisms, looking forward their application as lidocaine (LDC) drug-delivery systems in arthroplastic surgeries.We have studied the HA-micelle interaction using Dynamic Light Scattering (DLS), themicellization and sol-gel transition processes by Differential Scanning Calorimetry (DSC) and Rheology., of PL-based hydrogels and. The presence of HA provided the formation of larger micellar dimensions from ~26.0 to 42.4 nm. The incorporation of HA did not change the micellization temperatures and stabilized hydrogels rheological properties (G′ N G″), showing no interference on PLthermoreversible properties. Small-Angle-X-ray Scattering (SAXS) patterns revealed that HA incorporation effects were pronounced for PLF-127 and PLF-108 systems, showing transitions from lamellar to hexagonal phase organization (HA-PLF-127) and structural changes from cubic to gyroid and/or cubic to lamellar. The HA insertion effects were also observed on drug release profiles, since lower LDC release constants (Krel = 0.24–0.41 mM·h−1) were observed for HA-PLF-127, that presented a hexagonal phase organization. Furthermore, the HA-PL systems presented reduced in vitro cytotoxic effects, pointed out their tendency to selfassembly and possible application as drug delivery systems.
Palavras-Chave: hyaluronic acid; pluronics; hydrogels; anesthetics; calorimetry; toxicity; micellar systems; small angle scattering; drug delivery
. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. International Journal of Biological Macromolecules, v. 111, p. 1245-1254, 2018. DOI: 10.1016/j.ijbiomac.2018.01.064. Disponível em: http://repositorio.ipen.br/handle/123456789/28976. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. IAEA contribution to nanosized targeted radiopharmaceuticals for drug delivery. Pharmaceutics, v. 14, n. 5, p. 1-26, 2022. DOI: 10.3390/pharmaceutics14051060 Abstract: The rapidly growing interest in the application of nanoscience in the future design of radiopharmaceuticals and the development of nanosized radiopharmaceuticals in the late 2000′s, resulted in the creation of a Coordinated Research Project (CRP) by the International Atomic Energy Agency (IAEA) in 2014. This CRP entitled ‘Nanosized delivery systems for radiopharmaceuticals’ involved a team of expert scientist from various member states. This team of scientists worked on a number of cutting-edge areas of nanoscience with a focus on developing well-defined, highly effective and site-specific delivery systems of radiopharmaceuticals. Specifically, focus areas of various teams of scientists comprised of the development of nanoparticles (NPs) based on metals, polymers, and gels, and their conjugation/encapsulation or decoration with various tumor avid ligands such as peptides, folates, and small molecule phytochemicals. The research and development efforts also comprised of developing optimum radiolabeling methods of various nano vectors using diagnostic and therapeutic radionuclides including Tc-99m, Ga-68, Lu-177 and Au-198. Concerted efforts of teams of scientists within this CRP has resulted in the development of various protocols and guidelines on delivery systems of nanoradiopharmaceuticals, training of numerous graduate students/post-doctoral fellows and publications in peer reviewed journals while establishing numerous productive scientific networks in various participating member states. Some of the innovative nanoconstructs were chosen for further preclinical applications—all aimed at ultimate clinical translation for treating human cancer patients. This review article summarizes outcomes of this major international scientific endeavor.
Palavras-Chave: nanoparticles; drug delivery; radiopharmaceuticals; polymers; radioisotopes; theranostics
. IAEA contribution to nanosized targeted radiopharmaceuticals for drug delivery. Pharmaceutics, v. 14, n. 5, p. 1-26, 2022. DOI: 10.3390/pharmaceutics14051060. Disponível em: http://repositorio.ipen.br/handle/123456789/33200. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. In loco retention effect of magnetic core mesoporous silica nanoparticles doped with trastuzumab as intralesional nanodrug for breast cancer. Artificial Cells, Nanomedicine, and Biotechnology, v. 46, S3, p. S725-S733, 2018. DOI: 10.1080/21691401.2018.1508030 Abstract: Breast cancer is women’s most common type of cancer, with a global rate of over 522,000 deaths per year. One of the main problems related to breast cancer relies in the early detection, as the specialized treatment. In this direction was developed, characterized and tested in vivo a smart delivery system, based on radiolabelled magnetic core mesoporous silica doped with trastuzumab as intralesional nanodrug for breast cancer imaging and possible therapy. The results showed that nanoparticles had a size of 58.9 ± 8.1 nm, with specific surface area of 872 m2/g and pore volume of 0.85 cm3/g with a pore diameter of 3.15 nm. The magnetic core mesoporous silica was efficiently labelled with 99mTc (97.5% ±0.8) and doped >98%. The cytotoxicity assay, demonstrated they are safe to use. The data were corroborated with the IC50 result of: 829.6 mg ± 43.2. The biodistribution showed an uptake by the tumour of 7.5% (systemic via) and 97.37% (intralesional) with less than 3% of these nanoparticles absorbed by healthy tissues. In a period 6-h post-injection, no barrier delimited by the tumour was crossed, corroborating the use as intralesional nanodrug.
Palavras-Chave: neoplasms; mammary glands; nanoparticles; silica; magnetic cores; equipment; drugs; drug delivery; atomic force microscopy; radiochemical analysis
. In loco retention effect of magnetic core mesoporous silica nanoparticles doped with trastuzumab as intralesional nanodrug for breast cancer. Artificial Cells, Nanomedicine, and Biotechnology, v. 46, p. S725-S733, 2018. S3, DOI: 10.1080/21691401.2018.1508030. Disponível em: http://repositorio.ipen.br/handle/123456789/29945. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. In vitro and in vivo toxicology evaluation to determine suitable biomedical polymers for development of a papain-containing drug delivery system. Latin American Journal of Pharmacy, v. 30, n. 6, p. 1104-1108, 2011.
Palavras-Chave: papain; epidermis; cytology; toxicity; polymers; drug delivery; in vitro; in vivo
. In vitro and in vivo toxicology evaluation to determine suitable biomedical polymers for development of a papain-containing drug delivery system. Latin American Journal of Pharmacy, v. 30, n. 6, p. 1104-1108, 2011. Disponível em: http://repositorio.ipen.br/handle/123456789/4490. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artificial Cells, Nanomedicine, and Biotechnology, v. 46, S1, p. S1080–S1087, 2018. DOI: 10.1080/21691401.2018.1443941 Abstract: Cancer is responsible for more than 12% of all causes of death in the world, with an annual death rate of more than 7 million people. In this scenario melanoma is one of the most aggressive ones with serious limitation in early detection and therapy. In this direction we developed, characterized and tested in vivo a new drug delivery system based on magnetic core-mesoporous silica nanoparticle that has been doped with dacarbazine and labelled with technetium 99 m to be used as nano-imaging agent (nanoradiopharmaceutical) for early and differential diagnosis and melanoma by single photon emission computed tomography. The results demonstrated the ability of the magnetic core-mesoporous silica to be efficiently (>98%) doped with dacarbazine and also efficiently labelled with 99mTc (technetium 99 m) (>99%). The in vivo test, using inducted mice with melanoma, demonstrated the EPR effect of the magnetic core-mesoporous silica nanoparticles doped with dacarbazine and labelled with technetium 99 metastable when injected intratumorally and the possibility to be used as systemic injection too. In both cases, magnetic core-mesoporous silica nanoparticles doped with dacarbazine and labelled with technetium 99 metastable showed to be a reliable and efficient nano-imaging agent for melanoma.
Palavras-Chave: melanomas; neoplasms; silica; nanoparticles; magnetic properties; magnetic cores; drug delivery; therapy; images; technetium 99
. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artificial Cells, Nanomedicine, and Biotechnology, v. 46, p. S1080–S1087, 2018. S1, DOI: 10.1080/21691401.2018.1443941. Disponível em: http://repositorio.ipen.br/handle/123456789/29947. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Microemulsions formed by PPG-5-CETETH-20 at low concentrations for transdermal delivery of nifedipine: structural and in vitro study. Colloids and Surfaces B: Biointerfaces, v. 214, p. 1-9, 2022. DOI: 10.1016/j.colsurfb.2022.112474 Abstract: Nifedipine is a potent anti-hypertensive, which is poorly orally bioavailable on account of first-pass metabolism, short half-life, and low water solubility. This study aimed to develop a microemulsified system with low surfactant concentration and to evaluate the influence of microemulsion (ME) phase behavior on skin permeation of nifedipine, as drug model. Thereafter, MEs were obtained using PPG-5-CETETH-20, oleic acid, and phosphate buffer at pH 5.0. The selected MEs were isotropic, with droplet diameters less than 10 nm, polydispersity index < 0.25, and pH between 5.0 and 5.2. MEs presented low viscosity and Newtonian behavior. SAXS results confirmed bicontinuous and oil-in-water (o/w) MEs formation. The presence of the drug promoted only very slight modifications in the ME structure. The MEs presented ability to deliver nifedipine via the transdermal route when in comparison with the control. Nevertheless, the skin permeated and retained amounts from the o/w and bicontinuous formulations did not differ significantly. The ATR-FTIR demonstrated that both formulations promoted fluidization and disorganization of lipids and increased the drug diffusion and partition coefficients in the skin. In conclusion, PPG-5-CETETH-20 MEs obtained proved to be effective skin permeation enhancers, acting by rising the coefficients of partition and diffusion of the nifedipine in the skin.
Palavras-Chave: emulsions; nanotechnology; surfactants; drug delivery
. Microemulsions formed by PPG-5-CETETH-20 at low concentrations for transdermal delivery of nifedipine: structural and in vitro study. Colloids and Surfaces B: Biointerfaces, v. 214, p. 1-9, 2022. DOI: 10.1016/j.colsurfb.2022.112474. Disponível em: http://repositorio.ipen.br/handle/123456789/33167. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Microspheres for bone regeneration. In: FRACETO, LEONARDO F. (Ed.); ARAUJO, DANIELE R. de (Ed.). Microspheres: Technologies, Applications and Role in Drug Delivery Systems. Hauppauge, New York, USA: Nova Science Publishers, 2015. p. 161-180, cap. 6. Abstract: Nowadays, with global population aging and habits, cases of fracture, wear and bone loss have constantly concerned health authorities. As an alternative for the recovery of such patients and the promotion of better living conditions, there is a pressing need for development of biomaterials that can successfully replace or regenerate bone tissue. In this research area, microespheres deserve great attention due their unique properties. The specific material and the characteristics of the microspheres to be used for bone regeneration depend on each specific situation, taking into account aspects such as bone characteristics, injury size and selection of the most appropriate technique for such treatment. Generally, microspheres can act as a scaffold for the temporary or permanent filling of the injured area, or may act as a controlled drug delivery system. Moreover, the microspheres can simultaneously fulfill both functions. In this chapter, an overall view of bone tissue is presented, including the structure and functions. The main aspects of bone regeneration are briefly discussed, considering its fracture self-repairing mechanisms through reabsorption and deposition of the mineralized matrix process. When such mechanisms are not enough to provide full recovery, tissue regeneration strategies for such repairs are presented, highlighting the use of biomaterials and specifically microspheres. Finally, some examples of microsphere application to different situations related to the bone regeneration process are discussed.
Palavras-Chave: bone tissues; regeneration; biological regeneration; skeleton; microspheres; drug delivery
. Microspheres for bone regeneration. In: FRACETO, LEONARDO F. (ed.); ARAUJO, DANIELE R. de (ed.). Microspheres: Technologies, Applications and Role in Drug Delivery Systems. Hauppauge, New York, USA: Nova Science Publishers, 2015. cap. 6. p. 161-180. Disponível em: http://repositorio.ipen.br/handle/123456789/31161. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Mucoadhesive gellan gum-based and carboxymethyl cellulose -based hydrogels containing gemcitabine and papain for bladder cancer treatment. International Journal of Biological Macromolecules, v. 242, n. 3, p. 1-16, 2023. DOI: 10.1016/j.ijbiomac.2023.124957 Abstract: Local treatment of bladder cancer faces several limitations such as short residence time or low permeation through urothelium tissue. The aim of this work was to develop patient-friendly mucoadhesive gel formulations combining gemcitabine and the enzyme papain for improved intravesical chemotherapy delivery. Hydrogels based on two different polysaccharides, gellan gum and sodium carboxymethylcellulose (CMC), were prepared with either native papain or papain nanoparticles (nanopapain) to explore for the first time their use as permeability enhancers through bladder tissue. Gel formulations were characterized regarding enzyme stability, rheological behavior, retention on bladder tissue and bioadhesion, drug release properties, permeation capacity, and biocompatibility. After 90 days of storage, the enzyme loaded in the CMC gels retained up to 83.5 ± 4.9 % of its activity in the absence of the drug, and up to 78.1 ± 5.3 with gemcitabine. The gels were mucoadhesive and the enzyme papain showed mucolytic action, which resulted in resistance against washing off from the urothelium and enhanced permeability of gemcitabine in the ex vivo tissue diffusion tests. Native papain shortened lag-time tissue penetration to 0.6 h and enhanced 2-fold drug permeability All formulations demonstrated pseudoplastic behavior and no irritability. Overall, the developed formulations have potential as an upgraded alternative to intravesical therapy for bladder cancer treatment.
Palavras-Chave: hydrogels; papain; polysaccharides; drug delivery; bladder; neoplasms
. Mucoadhesive gellan gum-based and carboxymethyl cellulose -based hydrogels containing gemcitabine and papain for bladder cancer treatment. International Journal of Biological Macromolecules, v. 242, n. 3, p. 1-16, 2023. DOI: 10.1016/j.ijbiomac.2023.124957. Disponível em: http://repositorio.ipen.br/handle/123456789/34103. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Mucoadhesive polymers and their applications in drug delivery systems for the treatment of bladder cancer. Gels, v. 8, n. 9, p. 1-25, 2022. DOI: 10.3390/gels8090587 Abstract: Bladder cancer (BC) is the tenth most common type of cancer worldwide, affecting up to four times more men than women. Depending on the stage of the tumor, different therapy protocols are applied. Non-muscle-invasive cancer englobes around 70% of the cases and is usually treated using the transurethral resection of bladder tumor (TURBIT) followed by the instillation of chemotherapy or immunotherapy. However, due to bladder anatomy and physiology, current intravesical therapies present limitations concerning permeation and time of residence. Furthermore, they require several frequent catheter insertions with a reduced interval between doses, which is highly demotivating for the patient. This scenario has encouraged several pieces of research focusing on the development of drug delivery systems (DDS) to improve drug time residence, permeation capacity, and target release. In this review, the current situation of BC is described concerning the disease and available treatments, followed by a report on the main DDS developed in the past few years, focusing on those based on mucoadhesive polymers as a strategy. A brief review of methods to evaluate mucoadhesion properties is also presented; lastly, different polymers suitable for this application are discussed.
Palavras-Chave: adhesion; drug delivery; bladder; neoplasms; hydrogels; polymers; therapy
. Mucoadhesive polymers and their applications in drug delivery systems for the treatment of bladder cancer. Gels, v. 8, n. 9, p. 1-25, 2022. DOI: 10.3390/gels8090587. Disponível em: http://repositorio.ipen.br/handle/123456789/33418. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Poly(vinyl alcohol) (PVAl) and poly(N-2-vinyl-pyrrolidone) hydrogels nanostructured by laponite clay for drug delivery. Journal of Materials Science and Engineering B, v. 12, n. 7-9, p. 97-107, 2022. DOI: 10.17265/2161-6221/2022.7-9.003 Abstract: Hydrogels for wound dressings are usually developed for contact surfaces where mechanical properties are conveniently required. In this sense nanocomposite hydrogels based on PVAl (poly(vinyl alcohol) and PVP (poly(N-2-vinil-pirrolidone)) containing 0.5-1.5 wt% of the synthetic laponite RD clay were prepared by a gamma radiation process and compared with similar membranes composed separately of PVP or PVAl. This study aimed to evaluate the effect of clay on the properties and the differences of the polymer blend instead of a unique polymer. The morphology of the hydrogels was evaluated by spectrometric techniques using XRD (X-ray diffraction), SEM (scanning electron microscopy), swelling assay, and FTIR (infrared spectroscopy). The swelling kinetics at 22 °C and the mechanical properties by a tensile test comprised the structural properties that were assessed. The results showed PVA/PVP network depends directly on the clay concentration in the nanocomposite hydrogels. The blend PVP/PVAl proved to have potentially efficient mechanical properties for drug delivery in the treatment of wounds.
Palavras-Chave: drug delivery; clays; hydrogels; nanocomposites
. Poly(vinyl alcohol) (PVAl) and poly(N-2-vinyl-pyrrolidone) hydrogels nanostructured by laponite clay for drug delivery. Journal of Materials Science and Engineering B, v. 12, n. 7-9, p. 97-107, 2022. DOI: 10.17265/2161-6221/2022.7-9.003. Disponível em: http://repositorio.ipen.br/handle/123456789/33428. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A pre‑formulation study of tetracaine loaded in optimized nanostructured lipid carriers. Scientific Reports, v. 11, n. 1, p. 1-15, 2021. DOI: 10.1038/s41598-021-99743-6 Abstract: Tetracaine (TTC) is a local anesthetic broadly used for topical and spinal blockade, despite its systemic toxicity. Encapsulation in nanostructured lipid carriers (NLC) may prolong TTC delivery at the site of injection, reducing such toxicity. This work reports the development of NLC loading 4% TTC. Structural properties and encapsulation efficiency (%EE > 63%) guided the selection of three pre-formulations of different lipid composition, through a 23 factorial design of experiments (DOE). DLS and TEM analyses revealed average sizes (193–220 nm), polydispersity (< 0.2), zeta potential |− 21.8 to − 30.1 mV| and spherical shape of the nanoparticles, while FTIR-ATR, NTA, DSC, XRD and SANS provided details on their structure and physicochemical stability over time. Interestingly, one optimized pre-formulation (CP-TRANS/TTC) showed phase-separation after 4 months, as predicted by Raman imaging that detected lack of miscibility between its solid (cetyl palmitate) and liquid (Transcutol) lipids. SANS analyses identified lamellar arrangements inside such nanoparticles, the thickness of the lamellae been decreased by TTC. As a result of this combined approach (DOE and biophysical techniques) two optimized pre-formulations were rationally selected, both with great potential as drug delivery systems, extending the release of the anesthetic (> 48 h) and reducing TTC cytotoxicity against Balb/c 3T3 cells.
Palavras-Chave: anesthetics; nanostructures; carriers; lipids; drug delivery
. A pre‑formulation study of tetracaine loaded in optimized nanostructured lipid carriers. Scientific Reports, v. 11, n. 1, p. 1-15, 2021. DOI: 10.1038/s41598-021-99743-6. Disponível em: http://repositorio.ipen.br/handle/123456789/32392. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Pseudoboehmite as a drug delivery system for acyclovir. Scientific Reports, v. 11, n. 1, p. 1-12, 2021. DOI: 10.1038/s41598-021-94325-y Abstract: Herpes simplex virus is among the most prevalent sexually transmitted infections. Acyclovir is a potent, selective inhibitor of herpes viruses and it is indicated for the treatment and management of recurrent cold sores on the lips and face, genital herpes, among other diseases. The problem of the oral bioavailability of acyclovir is limited because of the low permeability across the gastrointestinal membrane. The use of nanoparticles of pseudoboehmite as a drug delivery system in vitro assays is a promising approach to further the permeability of acyclovir release. Here we report the synthesis of high purity pseudoboehmite from aluminium nitrate and ammonium hydroxide containing nanoparticles, using the sol–gel method, as a drug delivery system to improve the systemic bioavailability of acyclovir. The presence of pseudoboehmite nanoparticles were verified by infrared spectroscopy, transmission electron microscopy, and X-ray diffraction techniques. In vivo tests were performed with Wistar rats to compare the release of acyclovir, with and without the addition of pseudoboehmite. The administration of acyclovir with the addition of pseudoboehmite increased the drug content by 4.6 times in the plasma of Wistar rats after 4 h administration. We determined that the toxicity of pseudoboehmite is low up to 10 mg/mL, in gel and the dried pseudoboehmite nanoparticles.
Palavras-Chave: drug delivery; aluminium compounds; permeability; nanoparticles; viruses; herpes simplex
. Pseudoboehmite as a drug delivery system for acyclovir. Scientific Reports, v. 11, n. 1, p. 1-12, 2021. DOI: 10.1038/s41598-021-94325-y. Disponível em: http://repositorio.ipen.br/handle/123456789/32395. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Pseudoboehmite nanocarriers in cosmetic formulations. International Journal of Development Research, v. 11, n. 6, p. 47735-47738, 2021. DOI: 10.37118/ijdr.22018.06.2021 Abstract: The aim of this study is to contribute to the preparation and characterization of nanoemulsions for anti-aging cosmetic use. Palmarosa oil and Rosehip nanoemulsions were prepared with different active cosmetic ingredients such as mandelic acid and hyaluronic acid, in concentrations of 1%, 3% and 5% (wt%) of pseudoboehmite. After the nanoemulsions analysis, they were characterized in the following parameters: visual analysis, pH, density and optical microscopy. The results obtained show the possibility of using different compositions, the most suitable were: palmarosa oil nanoemulsion with 3 or 5wt% of mandellic acid/pseudoboehmite and palmarosa oil nanoemulsion with 1,3 or 5% of hyaluronic acid/pseudobohemite.
Palavras-Chave: consumer products; hyaluronic acid; mandelic acid; emulsions; drug delivery
. Pseudoboehmite nanocarriers in cosmetic formulations. International Journal of Development Research, v. 11, n. 6, p. 47735-47738, 2021. DOI: 10.37118/ijdr.22018.06.2021. Disponível em: http://repositorio.ipen.br/handle/123456789/32640. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
Itens para a visualização no momento 1-20 de 24
Buscar no repositório
Navegar
Minha conta
Visualizar
A pesquisa no RD utiliza os recursos de busca da maioria das bases de dados. No entanto algumas dicas podem auxiliar para obter um resultado mais pertinente.
✔ É possível efetuar a busca de um autor ou um termo em todo o RD, por meio do Buscar no Repositório , isto é, o termo solicitado será localizado em qualquer campo do RD. No entanto esse tipo de pesquisa não é recomendada a não ser que se deseje um resultado amplo e generalizado.
✔ A pesquisa apresentará melhor resultado selecionando um dos filtros disponíveis em Navegar
✔ Os filtros disponíveis em Navegar tais como: Coleções, Ano de publicação, Títulos, Assuntos, Autores, Revista, Tipo de publicação são autoexplicativos. O filtro, Autores IPEN apresenta uma relação com os autores vinculados ao IPEN; o ID Autor IPEN diz respeito ao número único de identificação de cada autor constante no RD e sob o qual estão agrupados todos os seus trabalhos independente das variáveis do seu nome; Tipo de acesso diz respeito à acessibilidade do documento, isto é , sujeito as leis de direitos autorais, ID RT apresenta a relação dos relatórios técnicos, restritos para consulta das comunidades indicadas.

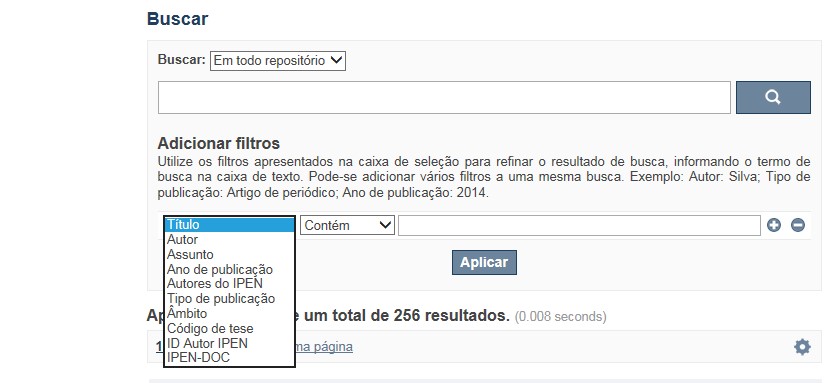
A opção Busca avançada utiliza os conectores da lógica boleana, é o melhor recurso para combinar chaves de busca e obter documentos relevantes à sua pesquisa, utilize os filtros apresentados na caixa de seleção para refinar o resultado de busca. Pode-se adicionar vários filtros a uma mesma busca.
Exemplo:
Buscar os artigos apresentados em um evento internacional de 2015, sobre loss of coolant, do autor Maprelian.
Autor: Maprelian
Título: loss of coolant
Tipo de publicação: Texto completo de evento
Ano de publicação: 2015
✔ Para indexação dos documentos é utilizado o Thesaurus do INIS, especializado na área nuclear e utilizado em todos os países membros da International Atomic Energy Agency – IAEA , por esse motivo, utilize os termos de busca de assunto em inglês; isto não exclui a busca livre por palavras, apenas o resultado pode não ser tão relevante ou pertinente.
✔ 95% do RD apresenta o texto completo do documento com livre acesso, para aqueles que apresentam o ![]() significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
✔ Ao efetuar a busca por um autor o RD apresentará uma relação de todos os trabalhos depositados no RD. No lado direito da tela são apresentados os coautores com o número de trabalhos produzidos em conjunto bem como os assuntos abordados e os respectivos anos de publicação agrupados.
✔ O RD disponibiliza um quadro estatístico de produtividade, onde é possível visualizar o número dos trabalhos agrupados por tipo de coleção, a medida que estão sendo depositados no RD.
✔ Na página inicial nas referências são sinalizados todos os autores IPEN, ao clicar nesse símbolo ![]() será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
✔ Na página do pesquisador, é possível verificar, as variações do nome, a relação de todos os trabalhos com texto completo bem como um quadro resumo numérico; há links para o Currículo Lattes e o Google Acadêmico ( quando esse for informado).
ATENÇÃO!
ESTE TEXTO "AJUDA" ESTÁ SUJEITO A ATUALIZAÇÕES CONSTANTES, A MEDIDA QUE NOVAS FUNCIONALIDADES E RECURSOS DE BUSCA FOREM SENDO DESENVOLVIDOS PELAS EQUIPES DA BIBLIOTECA E DA INFORMÁTICA.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
1. Portaria IPEN-CNEN/SP nº 387, que estabeleceu os princípios que nortearam a criação do RDI, clique aqui.
2. A experiência do Instituto de Pesquisas Energéticas e Nucleares (IPEN-CNEN/SP) na criação de um Repositório Digital Institucional – RDI, clique aqui.
O Repositório Digital do IPEN é um equipamento institucional de acesso aberto, criado com o objetivo de reunir, preservar, disponibilizar e conferir maior visibilidade à Produção Científica publicada pelo Instituto, desde sua criação em 1956.
Operando, inicialmente como uma base de dados referencial o Repositório foi disponibilizado na atual plataforma, em junho de 2015. No Repositório está disponível o acesso ao conteúdo digital de artigos de periódicos, eventos, nacionais e internacionais, livros, capítulos, dissertações, teses e relatórios técnicos.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
