Navegação Periódicos - Resumos por assunto "neoplasms"
- Página inicial
- →
- IPEN
- →
- Periódicos - Resumos
- →
- Navegação Periódicos - Resumos por assunto
- Sobre
- Perfil Técnico
- Política de funcionamento
- Ajuda
- Apresentação
Navegação Periódicos - Resumos por assunto "neoplasms"
Itens para a visualização no momento 1-20 de 29
-
. 111In-DTPA-octreotide: production and quality control. Radiologia Brasileira, v. 39, n. Suppl., 2, p. p. 93, 2006.
Palavras-Chave: indium 111; labelled compounds; neoplasms; diagnosis; computerized tomography; quality control
. 111In-DTPA-octreotide: production and quality control. Radiologia Brasileira, v. 39, n. Suppl., 2, p. p. 93, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8760. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. 177Lu-PSMA-617: Brazilian experience. Journal of Nuclear Medicine, v. 60, supplement 1 200, 2019. Abstract: Introduction: PSMA-617 radiolabeled with lutetium-177 has shown good results in compassionate studies around the world, and there is great interest in this kind of therapy in Brazil. The Nuclear and Research Institute (IPEN-CNEN) in São Paulo city, is a national radiopharmaceutical producer and the distribution of radiopharmaceuticals for therapy in a country of continental dimensions such as Brazil, becomes a challenge from the standpoint of guaranteeing the stability of the product. This work evaluated the scheduling of pilot batches for the production of 177Lu-PSMA-617 and studied the effect of dilution and freezing on the stability of mono-doses of the product. Materials and Methods: Radiolabeling of PSMA-617 (ABX, Germany) with lutetium-177 (JSC, Russia) was performed in heating block at 90 °C for 30 minutes, 37 GBq (1 Ci), 500 μg of peptide and sodium ascorbate (0,5 M pH 4,7) as buffer. At the end of the radiolabel, 0,5 mL of DTPA solution (4 mg/mL pH 4,5) was added and the product was diluted with an appropriate volume of saline solution 0,9%. The final product was filtrated in 0.22 membrane and the doses were fractionated (7,4 GBq calibrated for 24 hours in approximately 2,4 mL) and conditioned in appropriate lead chambers inside a dry ice bucket for transport simulation. The radiochemical purity (RP) was evaluated in a stability study at, 24 and 48 hours by TLC and HPLC. The radionuclidic purity, sterility and bacterial endotoxins were also evaluated. Results and discussion: The radiopharmaceutical was stable after 48 hours (99.47% TLC and 99.39% HPLC), and was approved in radionuclidic, sterility and endotoxins assays. The vials fractionated in mono-doses calibrated for 24 hours showed high stability through freezing and dilution, which allowed the transportation to the Cancer Hospital of Barretos, distant 420 km from the production center in São Paulo. All these mono-doses were administrated to the patients after 24 hours of the production. Also, these results denote that is possible to scale up this production until 74 GBq and be used in a clinical trial that is being planned. Conclusions: These carried out experiments demonstrated that it is possible to produce 177Lu-PSMA-617 for use in clinical trials in Brazil. Until now, four patients are under treatment (compassionate use). Also, these results demonstrate that it is still possible to increase production activity to 74 GBq, just like is normally done with 177Lu-DOTATATE.
Palavras-Chave: lutetium 177; radiopharmaceuticals; prostate; neoplasms; brazil
. 177Lu-PSMA-617: Brazilian experience. Journal of Nuclear Medicine, v. 60, 2019. supplement 1 200. Disponível em: http://repositorio.ipen.br/handle/123456789/31066. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Analysis of 10,327 pre-treatment ultrasound localizations for 387 prostate cancer patients treated with conformal 3D external beam radiation therapy. Medical Physics, v. 33, n. 6, p. 2026, 2006.
Palavras-Chave: prostate; neoplasms; radiotherapy; two-dimensional calculations; ultrasonography; external irradiation
. Analysis of 10,327 pre-treatment ultrasound localizations for 387 prostate cancer patients treated with conformal 3D external beam radiation therapy. Medical Physics, v. 33, n. 6, p. 2026, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8781. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Avaliação do potencial citotóxico da 2-tetradecilciclobutana em células hepáticas linhagem HepG2: estudos in vitro. Anais da SBBN, v. 3, p. 43-43, 2016. Abstract: Introdução: A irradiação de alimentos é um método eficaz e seguro para a preservação e armazenamento em longo prazo, é aprovado para utilização em mais de 60 países para diversas aplicações em uma ampla variedade de produtos alimentares (Agric. Food Chem. 51; 927, 2003. Food Chem. 201; 52-58, 2016). Este processo é realizado através da utilização de feixes de elétrons acelerados, raios-X ou radiação gama (60Co ou 137Cs). As 2-Alcilciclobutanonas (2-ACBs) são os únicos produtos radiolíticos conhecidos gerados a partir de alimentos que possuem ácidos graxos (Triglicérides) e são submetidos à irradiação (J. Food Protc. 67; 142, 2004. T. Food Scie.Tech. 44; 66-78, 2015). O ácido analisado neste estudo é o esteárico que quando irradiado forma 2-Tetradecilciclobutanona (2-tDCB). Desde a década de 1990 estudos toxicológicos de segurança das 2-ACBs tem sido conduzido extensivamente através de compostos sintéticos. Testes de mutagenicidade das 2-ACBs realizados indicam claramente que nenhuma evidência foi observada, enquanto estudos de viabilidade apresentaram citotoxicidade notada através da morte celular (Food Scie. Tech. 44; 66-78, 2015). Parte das 2-ACBs ingeridas é excretada através das fezes e parte ficam depositadas em tecidos adiposos. Estudos realizados até o momento foram somente em células de cólon. A linhagem escolhida para este trabalho é derivada de células hepáticas uma vez que o acumulo de gordura neste órgão é bastante comum. Objetivo: Avaliar possíveis danos citotóxicos, através do teste de viabilidade celular MTT observando a influência de diversas concentrações da 2-tDCB em diferentes tempos de incubação em células hepáticas da linhagem HepG2. Métodos: O composto 2-tDCB foi solubilizado em etanol a 2%. A linhagem celular escolhida é derivada de hepatocarcinoma humano (HepG2) e foi cultivada em meio de cultura suplementado com 10% de soro fetal bovino. As células foram plaqueadas na densidade de 5x103 cél/poço em uma placa de 96 poços. O efeito citotóxico da 2-tDCB foi avaliado nas concentrações de 100, 300 e 500μM, durante 24 e 48 horas. Os testes foram realizados de acordo com instruções do kit CellTiter96 Aqueus Non-radioactive Cell Proliferation Assay, em triplicatas (biológica e experimental) e os resultados foram analisados pelo programa Prisma GraphPad. Resultados: A linhagem tratada com 2-tDCB em 24 e 48h não apresentou citotoxicidade em nenhuma das concentrações avaliadas. Conclusão: Não houve inviabilidade causada pelo composto 2-tDCB na linhagem de células hepática estudadas, nenhum dano foi observado em nenhuma das variações pesquisadas. Estudos mais aprofundados são necessários para identificar os mecanismos moleculares pela qual o composto em questão atua.
Palavras-Chave: carboxylic acids; carcinomas; irradiation; liver cells; radiation effects; toxicity; viability; animal cells; diseases; neoplasms; organic acids; somatic cells
. Avaliação do potencial citotóxico da 2-tetradecilciclobutana em células hepáticas linhagem HepG2: estudos in vitro. Anais da SBBN, v. 3, p. 43-43, 2016. Disponível em: http://repositorio.ipen.br/handle/123456789/31152. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Avoiding chemotherapy resistance in squamous cell carcinomas: anticancer activities of terpenoids and their impact on the regulation of microRNAs. Cancer Research, v. 77, 13 Supplement, 2017. DOI: 10.1158/1538-7445.AM2017-5456 Abstract: MicroRNAs are small noncoding RNAs that play important roles in cellular biology. They have been implicated in pharmacogenomics by down-regulating genes that are essential for drug function. In this work we verified the potential anticancer activity of the quinone methide triterpenes maytenin and 22-β-hydroxymaytenin, as well as of a quinone methide triterpene-rich extract obtained from cultivated Maytenus ilicifolia root cells, and evaluated the associated microRNA expression following half maximal inhibitory concentration (IC50) treatment. Standard selectivity index (SI) for the isolated compounds and the root cell extract was determined by the logarithmic shift in effective concentration (IC50) between cancer cell lines and oral keratinocytes. Both isolated molecules as well as the root cell extract presented pronounced antiproliferative and pro-apoptotic activities in four cell lines derived from head and neck squamous cell carcinomas, including a metastasis-derived cell line. A positive SI, with an average 2-fold increase in potency, was detected for single agents and for the extract. MicroRNA expression profiles were assessed at 24h, 48h and 72h following treatment and an average of 100 molecules presented consistent marked variation in expression levels. Considering associations of microRNAs, genes they regulate, and the drugs effects dependent on these genes, the down-regulation of miR-193a-3p and miR-21 in treated cells is of particular interest. Both microRNAs have been involved in 5-fluorouracil and cisplatin resistance, current agents of standard chemoradiotherapy for locally advanced head and neck cancer. Squamous cell carcinoma of the head and neck is one of the most common cancer types worldwide whereas treatment options based on conventional therapies or targeted therapies under development have limited efficacy. Plant-derived products are valuable sources for the development of new therapeutic options for cancer treatment or as synergistic agents in existing regular care.
Palavras-Chave: chemotherapy; neoplasms; epithelium; histology; rna; inhibition
. Avoiding chemotherapy resistance in squamous cell carcinomas: anticancer activities of terpenoids and their impact on the regulation of microRNAs. Cancer Research, v. 77, 2017. 13 Supplement, DOI: 10.1158/1538-7445.AM2017-5456. Disponível em: http://repositorio.ipen.br/handle/123456789/31338. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Brazil radioactive sources production for cancer treatment. Medical Physics International, v. 4, n. 2, p. 237-237, 2016. Abstract: The modality, known as brachytherapy, was performed in Brazil by only a hand full of hospitals at an extremely high cost. For producing new sources, five major areas must be considered: 1) source production: nuclear activation and/or radiochemical reaction; 2) welding; 3) Quality control: leakage tests; 4) Dosimetry and metrology; 5) Operational procedures; 6) validation studies. To perform all steps, a multidisciplinary team works together to overcome difficulties. - Iridium-192 pellets: In Brazil there are 140 machines with pellets that replacement every 5 years. Our new production line has assembly, welding and quality control hot cells. - Iridium-192 wires: Produced since 1999. The wire is activated at IPENs IEA-R1 reactor for 30 hours with 5x1013 n/cm-2.s-1 neutron flux resulting in 192 mCi maximum activity. - Iridium-192 seed: New seed for ophthalmic cancer treatment. The irradiation device presented 90% activity homogeneity. We are still testing in-vivo. - Iodine-125 seeds: Largely used in low dose brachytherapy. I-125 binding yield achieved with our new reaction was 80%; Laser welding presented 70% efficiency. Approved in all leakage tests. - Other ongoing projects: Veterinary brachytherapy, Waste management, Radionecrosis healing with laser, calibrations sources production, linear accelerator calculations for hospitals, sources with polymeric matrix Our Iodine-125 seeds will be available in 2018. All other projects are advancing. We will continue to develop new products hoping to help the Brazilian population fight against cancer. For producing new sources, five major areas must be considered: 1) source production: nuclear activation and/or radiochemical reaction; 2) welding; 3) Quality control:eakage tests; 4) Dosimetry and metrology; 5) Operational procedures; 6) validation studies. To perform all steps, a multidisciplinary team works together to overcome difficulties
Palavras-Chave: irradiation devices; ionizing radiations; brachytherapy; neoplasms; iodine 125; iridium 192; radiation sources; brazil
. Brazil radioactive sources production for cancer treatment. Medical Physics International, v. 4, n. 2, p. 237-237, 2016. Disponível em: http://repositorio.ipen.br/handle/123456789/28170. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Comparative pharmacokinetic and biodistribution studies of two novel [sup(177)Lu]bombesin analogues for prostate cancer target therapy. European Journal of Nuclear Medicine and Molecular Imaging, v. 35, p. 322, 2008.
Palavras-Chave: neoplasms; prostate; radioisotopes; kinetics; radiopharmaceuticals; labelling; lutetium 177; radiotherapy
. Comparative pharmacokinetic and biodistribution studies of two novel [sup(177)Lu]bombesin analogues for prostate cancer target therapy. European Journal of Nuclear Medicine and Molecular Imaging, v. 35, p. 322, 2008. Disponível em: http://repositorio.ipen.br/handle/123456789/8812. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Comparative uptake of Tc-99m-MIBI and Tc-99m-N- acetylcysteine in an experimental tumor model. Journal of Nuclear Medicine, v. 39, n. 5, p. 221P, 1998.
Palavras-Chave: labelling; isonitriles; technetium 99; neoplasms; uptake; rats; organs; animal tissues; radiopharmaceuticals
. Comparative uptake of Tc-99m-MIBI and Tc-99m-N- acetylcysteine in an experimental tumor model. Journal of Nuclear Medicine, v. 39, n. 5, p. 221P, 1998. Disponível em: http://repositorio.ipen.br/handle/123456789/8797. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Comparison of two ionization chamber used absolute dose for intensity modulated radiotherapy of prostate quality assurance. Radiotherapy and Oncology, v. 76, Suppl. 2, p. S185, 2005.
Palavras-Chave: prostate; neoplasms; radiotherapy; ionization chambers; radiation doses; phantoms; quality assurance
. Comparison of two ionization chamber used absolute dose for intensity modulated radiotherapy of prostate quality assurance. Radiotherapy and Oncology, v. 76, p. S185, 2005. Suppl. 2. Disponível em: http://repositorio.ipen.br/handle/123456789/8868. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Effects of photobiomodulation on breast tumor- bearing mice before radiotherapy. Lasers in Medical Science, v. 35, n. 1, p. 269-269, 2020. DOI: 10.1007/s10103-019-02900-7 Abstract: Photobiomodulation (PBM) has been studied to modify the cellular response to ionizing radiation. However, its combination with radiotherapy (RT) has not been reported in cancer treatment. The aim of this study was to evaluate the effects of PBM applied before RT on breast tumor-bearing mice. Female BALB/c mice were inoculated with breast 4T1 cells into mammary fat pad and divided into 4 groups (n =5 per group): control (with no treatment), only RT, and PBM combined to RT in two different protocols. RT was locally applied using a 60Co source with dose of 60 Gy in fractions of 15 Gy. For PBM, a red laser (660 nm, 500 mW/cm2) was used in two regimes: single exposure 24 h before RT (fluence of 150 J/cm2) and immediately before each RT session (fluence of 37.5 J/cm2 per session). After treatment, tumor volume, platelets, white and red blood cell levels were evaluated during 14 days. Our results showed no statistically significant differen ces in t umor volume, platelet and red blood cell levels comparing control, RT and PBM+RT groups. However, PBM was able to sustain normal white blood cell levels compared to RT and control groups. In addition, mice that received PBM concomitant with RT presented a longer survival. In fact, for this group only 12.5 % of the animals died during experimental period. These findings indicate that PBM could be combined to RT to provide therapeutic anti-cancer benefits.
Palavras-Chave: ionizing radiations; therapy; neoplasms; tumor cells; mice; mammary glands; radiotherapy
. Effects of photobiomodulation on breast tumor- bearing mice before radiotherapy. Lasers in Medical Science, v. 35, n. 1, p. 269-269, 2020. DOI: 10.1007/s10103-019-02900-7. Disponível em: http://repositorio.ipen.br/handle/123456789/31111. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Efficacy of photobiomodulation therapy in mitigating skin radiation damage. Lasers in Surgery and Medicine, v. 50, 29, p. S15-S16, 2018. DOI: 10.1002/lsm.22799 Abstract: Background: The use of sophisticated radiation dose delivery and fractionation has significantly improved cancer care. One of these involves localized, sustained ionizing dose delivery termed brachytherapy. Despite it therapeutic efficacy, specific side effects of brachytherapy include localized skin damage and breakdown for which only palliative treatments are currently available. The use of low dose biophotonics treatments to promote tissue healing is termed photobiomodulation (PBM) therapy. The aim of this study was to evaluate efficacy and molecular pathways of PBM therapy using two common wavelengths, red and near-infrared (NIR) to treat radiation wounds in athymic mice subjected to brachytherapy (sustained ionizing radiation from 125I seeds). Study Design/Materials and Method: A pilot study was performed with thirty-six athymic mice were accomplished for 60 days and divided into six groups: Surgical Control Group (No radiation and no PBM treatments); Radiation Control Group (125I seed 0.4252 mCi, no PBM); NIR-PBM Control Group (NIR PBM alone, LED at l¼880 nm); Red-PBM Control Group (Red PBM alone LED at l¼660 nm); Radiation- NIR PBM Group; Radiation-Red PBM Group. Following 21 days, radiation-induced wounds are evident. PBM treatments (both wavelengths with output power 40mW for 20 s, fluence 20 J/cm2 on top of implantation site) were performed every week up to 60 days. Wounds were evaluated every 7 days digital imaging, Laser Doppler Flowmetry (LDF) and tissue temperature with a thermographic camera. We also performed mPET-CT imaging using radioactive fluorodeoxyglucose (18F-FDG) at 51 and 81 days post-implantation. Animals were sacrifices progressively at each time point to correlate clinical observations with imaging and molecular tissue analyses. Tissues were collected to analyze molecular pathways correlating with inflammation, immune response, wound healing and angiogenesis using mRNA (qRT-PCR) and protein expression (immunostaining). Results: Both PBM treated groups demonstrated significant (p<0.05) improvements in skin radiation wound healing as compared to radiation group. Distinct improvements in clinical wound size and closure, improved tissue perfusion and reduced inflammation as evidenced by decreased wound thermal images. These wounds were also noted to have significant differences in the cytokine profiles (TGF-b, VEGF and PDGF) correlating with better healing responses. Radiation damage reduces brown fat composition that can potentially contribute to additional radiation-associated morbidities. The mPET-CT imaging noted significant preservation of brown fat composition in PBM-treated radiation alone groups. Further validation of these pathways is ongoing. Conclusion: Within the parameters of this study, PBM treatments demonstrated improved healing in radiation wounds due to ionizing radiation from 125I seeds. Ongoing work is examining the precise molecular pathways contributing to these therapeutic benefits. It is hoped this study will enable further development of this innovative therapy for managing side- effects from radiation treatments.
Palavras-Chave: neoplasms; brachytherapy; radiation doses; radiation injuries; wounds; biological recovery
. Efficacy of photobiomodulation therapy in mitigating skin radiation damage. Lasers in Surgery and Medicine, v. 50, p. S15-S16, 2018. 29, DOI: 10.1002/lsm.22799. Disponível em: http://repositorio.ipen.br/handle/123456789/29061. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Evaluation of the absorbed dose from patients based on whole-body 131I clearance in thyroid cancer therapy. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 89, 2006.
Palavras-Chave: patients; thyroid; neoplasms; radiotherapy; iodine 131; radiation doses; whole-body counting; radiation protection
. Evaluation of the absorbed dose from patients based on whole-body 131I clearance in thyroid cancer therapy. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 89, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8755. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. A gas chromatography technique for analysis of residual solvents in 18F-FDG preparation. Radiologia Brasileira, v. 39, n. Suppl., 2, p. p. 92, 2006.
Palavras-Chave: gas chromatography; solvents; fluorine 18; fluorodeoxyglucose; neoplasms
. A gas chromatography technique for analysis of residual solvents in 18F-FDG preparation. Radiologia Brasileira, v. 39, n. Suppl., 2, p. p. 92, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8759. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. In house production of 99mTc-EDDA-hynic-[TYR3]-octreotide for somatostatin receptor scintigraphy. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 84, 2006.
Palavras-Chave: scintiscanning; indium 111; dtpa; labelling; endocrine diseases; neoplasms; images
. In house production of 99mTc-EDDA-hynic-[TYR3]-octreotide for somatostatin receptor scintigraphy. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 84, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8753. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Invasive evaluation of 99mTc(CO)3-thymidine analog in a lung cancer model. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 93-94, 2006.
Palavras-Chave: technetium 99; thymidine; radiopharmaceuticals; labelled compounds; neoplasms; images; cell proliferation; lungs; mice
. Invasive evaluation of 99mTc(CO)3-thymidine analog in a lung cancer model. Radiologia Brasileira, v. 39, n. Suppl., 2, p. 93-94, 2006. Disponível em: http://repositorio.ipen.br/handle/123456789/8761. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Mass influence of DOTA-Rituximab in the radiolabelling with Lu-177. ALASBIMN Journal, v. ano 14, n. 54, 2011.
Palavras-Chave: radiopharmaceuticals; lutetium 177; labelling; hodgkins disease; monoclonal antibodies; purification; radiochemical analysis; radioimmunotherapy; neoplasms; metastases
. Mass influence of DOTA-Rituximab in the radiolabelling with Lu-177. ALASBIMN Journal, v. ano 14, n. 54, 2011. Disponível em: http://repositorio.ipen.br/handle/123456789/8892. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. Methodology for in vivo dosimetry using TLD-100 for radiotherapic treatment. Medical Physics, v. 44, n. 6, p. 2898-2899, 2017. DOI: 10.1002/mp.12304 Abstract: Cancer is a public health problem that affects approximately 27 million people worldwide. The most common type in Brazil among men is prostate cancer with 61 thousand cases. There are two forms of radiotherapy treatments that can be used: teletherapy and brachytherapy. Before starting the teletherapy treatment, a planning is done that makes the acquisition of the anatomical information of the patient to then classify the areas of interest. Dosimetry is performed as a quality control to ensure that the calculated dose is equal to that received by the patient. In vivo dosimetry acts as an independent measurement and this work aims at comparing the dosimetry performed using thermoluminescent dosimeters (LiF: Mg, Ti - TLD - 100) with dose values calculated in the planning system (TPS). Methods: All dosimeters were prepared to be used in an anthropomorphic phantom. A selection of dosimeters, 50 micro TLD’s, selected after heat treatment, were then irradiated and a reading was made. A case planned by TPS was selected and compared the dosimetry performed in an anthropomorphic phantom for the same case. Results: All values obtained were within the deviation ( 5%) allowed by the protocol. The results of this work will help to implement a new quality program in the Radiotherapy Service at Hospital das Cl ınicas de S~ao Paulo. Conclusion: The accurate dosimeter selection provided a feasible and reliable evaluation that enabled the comparison.
Palavras-Chave: neoplasms; prostate; radiotherapy; brachytherapy; thermoluminescent dosimetry
. Methodology for in vivo dosimetry using TLD-100 for radiotherapic treatment. Medical Physics, v. 44, n. 6, p. 2898-2899, 2017. DOI: 10.1002/mp.12304. Disponível em: http://repositorio.ipen.br/handle/123456789/31134. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. New core configuration for producing Iodine 125 seeds. Medical Physics, v. 44, n. 6, p. 2828-2829, 2017. DOI: 10.1002/mp.12304 Abstract: Purpose: Cancer is one of the most complex public health problems. Prostate cancer is the second most common among men. In prostate brachytherapy use Iodine-125, which is fixated on a silver substrate, then inserted and sealed in a titanium capsule. This work proposes a new source configuration using epoxy resin substrate. Methods: Comparation and analysis methods were used to define the methodology for combining iodine-125 in polymers. The parameters were immersion time, reaction type, concentration of the adsorption solution, specific activity of the radioactive solution, need for carrier and chemical form of radioactive iodine. Results: The methodology developed with an epoxy resin was very good. The final radioactive intake on the resin was higher than 80%. The immobilization of the radioactive solution occurred in the matrix, without any loss or deposition of undesirable materials on its surface, as evidenced by the smear test. The material maintains its integrity when autoclaved at 140 °C. The curing process of the resin was 40 minutes. With the value of the initial activity of the Iodine solution by mass (774.2 lCi/g), it was possible to calculate the immobilization efficiency Average of 680 lCi/g. The immersion test in distilled water at room temperature did not exceed the limit allowed by ISO 9978, which is 5 nCi (185 Bq), proof of no leakage. In a computational simulation by the Monte Carlo Method, PENELOPE, the simulations were consistent with the values adopted by the literature for the GE Healthcare model 6711, which shows the value of the dose rate constant as 0, 965 cGy.U-1.h-1. Conclusion: The effective method for combining iodine-125 in epoxy resin was determinated. The major advantage was the high efficiency percentage fixation, around 82,1 3,2%, and the simplicity and safety of the process.
Palavras-Chave: neoplasms; prostate; iodine 125; polymers; epoxides; resins
. New core configuration for producing Iodine 125 seeds. Medical Physics, v. 44, n. 6, p. 2828-2829, 2017. DOI: 10.1002/mp.12304. Disponível em: http://repositorio.ipen.br/handle/123456789/31135. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. New gold-198 nanoparticle synthesis to be used in cancer treatment. Medical Physics, v. 45, n. 6, p. e243-e243, 2018.
Palavras-Chave: aqua regia; brachytherapy; citrates; decay; gold 198; high-purity ge detectors; light scattering; nanoparticles; neoplasms; neutron flux; sodium compounds; synthesis; therapy
. New gold-198 nanoparticle synthesis to be used in cancer treatment. Medical Physics, v. 45, n. 6, p. e243-e243, 2018. Disponível em: http://repositorio.ipen.br/handle/123456789/29844. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
-
. New methodology for binding Iodine-125 onto silver for brachytherapy sources manufacture. Medical Physics, v. 44, n. 6, p. 2828-2828, 2017. DOI: 10.1002/mp.12304 Abstract: Purpose: Cancer is a major health care problem in Brazil and the world. The Brazil’s National Institute for Cancer estimates around 60,000 new prostate cancer cases for 2017. We are assembling a laboratory for production of iodine-125 sources used in prostate brachytherapy in Brazil, since the imported treatment is extremely expensive, thus only available in the private healthcare sector. There are several challenges when developing a laboratory to produce radioactive sources. From choosing a prototype to radiation safety, the task is enormous. The whole production line is full of new process and innovations. Among those, a new chemical reaction that deposit iodine-125 onto silver (core) was developed. This paper presents a new reaction for binding iodine-125 into a silver core. The fixation percentage was calculated by measuring the activity in an ionization chamber. This methodology will be implemented at the iodine-125 sources manufacture laboratory. Methods: Silver cores are washed with an etching solution (100% sulfuric acid) for 5 minutes with sonication. The cores were then placed in sodium sulfate for at least 3 days. They went from a silver matte to a black color. The reaction was allowed to proceed overnight. Each core was individually measured. Results: The yield was 69.2% 7.1%. Considering the silver attenuation is around 20% the results were consider satisfactory. Conclusion: By maximize the reaction yield, we will be able to generate a less costly product that will be available through our public healthcare.
Palavras-Chave: brachytherapy; iodine 125; silver; neoplasms; therapy
. New methodology for binding Iodine-125 onto silver for brachytherapy sources manufacture. Medical Physics, v. 44, n. 6, p. 2828-2828, 2017. DOI: 10.1002/mp.12304. Disponível em: http://repositorio.ipen.br/handle/123456789/31136. Acesso em: $DATA.Como referenciar este itemEsta referência é gerada automaticamente de acordo com as normas do estilo IPEN/SP (ABNT NBR 6023) e recomenda-se uma verificação final e ajustes caso necessário.
Itens para a visualização no momento 1-20 de 29
Buscar no repositório
Navegar
Minha conta
Visualizar
A pesquisa no RD utiliza os recursos de busca da maioria das bases de dados. No entanto algumas dicas podem auxiliar para obter um resultado mais pertinente.
✔ É possível efetuar a busca de um autor ou um termo em todo o RD, por meio do Buscar no Repositório , isto é, o termo solicitado será localizado em qualquer campo do RD. No entanto esse tipo de pesquisa não é recomendada a não ser que se deseje um resultado amplo e generalizado.
✔ A pesquisa apresentará melhor resultado selecionando um dos filtros disponíveis em Navegar
✔ Os filtros disponíveis em Navegar tais como: Coleções, Ano de publicação, Títulos, Assuntos, Autores, Revista, Tipo de publicação são autoexplicativos. O filtro, Autores IPEN apresenta uma relação com os autores vinculados ao IPEN; o ID Autor IPEN diz respeito ao número único de identificação de cada autor constante no RD e sob o qual estão agrupados todos os seus trabalhos independente das variáveis do seu nome; Tipo de acesso diz respeito à acessibilidade do documento, isto é , sujeito as leis de direitos autorais, ID RT apresenta a relação dos relatórios técnicos, restritos para consulta das comunidades indicadas.

A opção Busca avançada utiliza os conectores da lógica boleana, é o melhor recurso para combinar chaves de busca e obter documentos relevantes à sua pesquisa, utilize os filtros apresentados na caixa de seleção para refinar o resultado de busca. Pode-se adicionar vários filtros a uma mesma busca.
Exemplo:
Buscar os artigos apresentados em um evento internacional de 2015, sobre loss of coolant, do autor Maprelian.
Autor: Maprelian
Título: loss of coolant
Tipo de publicação: Texto completo de evento
Ano de publicação: 2015
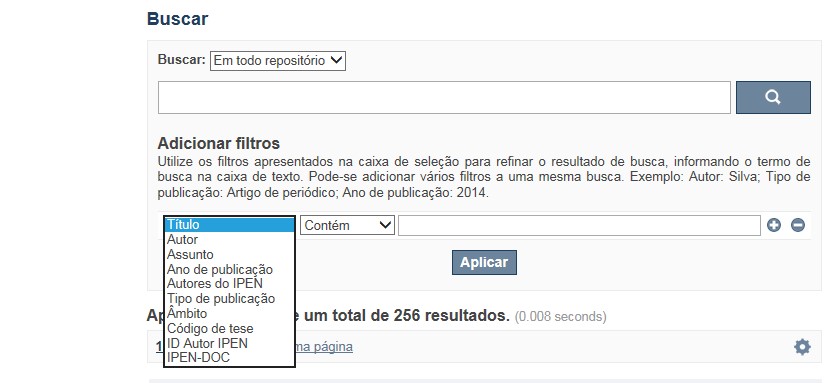
✔ Para indexação dos documentos é utilizado o Thesaurus do INIS, especializado na área nuclear e utilizado em todos os países membros da International Atomic Energy Agency – IAEA , por esse motivo, utilize os termos de busca de assunto em inglês; isto não exclui a busca livre por palavras, apenas o resultado pode não ser tão relevante ou pertinente.
✔ 95% do RD apresenta o texto completo do documento com livre acesso, para aqueles que apresentam o ![]() significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
significa que e o documento está sujeito as leis de direitos autorais, solicita-se nesses casos contatar a Biblioteca do IPEN,
bibl@ipen.br
.
✔ Ao efetuar a busca por um autor o RD apresentará uma relação de todos os trabalhos depositados no RD. No lado direito da tela são apresentados os coautores com o número de trabalhos produzidos em conjunto bem como os assuntos abordados e os respectivos anos de publicação agrupados.
✔ O RD disponibiliza um quadro estatístico de produtividade, onde é possível visualizar o número dos trabalhos agrupados por tipo de coleção, a medida que estão sendo depositados no RD.
✔ Na página inicial nas referências são sinalizados todos os autores IPEN, ao clicar nesse símbolo ![]() será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
será aberta uma nova página correspondente à aquele autor – trata-se da página do pesquisador.
✔ Na página do pesquisador, é possível verificar, as variações do nome, a relação de todos os trabalhos com texto completo bem como um quadro resumo numérico; há links para o Currículo Lattes e o Google Acadêmico ( quando esse for informado).
ATENÇÃO!
ESTE TEXTO "AJUDA" ESTÁ SUJEITO A ATUALIZAÇÕES CONSTANTES, A MEDIDA QUE NOVAS FUNCIONALIDADES E RECURSOS DE BUSCA FOREM SENDO DESENVOLVIDOS PELAS EQUIPES DA BIBLIOTECA E DA INFORMÁTICA.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
1. Portaria IPEN-CNEN/SP nº 387, que estabeleceu os princípios que nortearam a criação do RDI, clique aqui.
2. A experiência do Instituto de Pesquisas Energéticas e Nucleares (IPEN-CNEN/SP) na criação de um Repositório Digital Institucional – RDI, clique aqui.
O Repositório Digital do IPEN é um equipamento institucional de acesso aberto, criado com o objetivo de reunir, preservar, disponibilizar e conferir maior visibilidade à Produção Científica publicada pelo Instituto, desde sua criação em 1956.
Operando, inicialmente como uma base de dados referencial o Repositório foi disponibilizado na atual plataforma, em junho de 2015. No Repositório está disponível o acesso ao conteúdo digital de artigos de periódicos, eventos, nacionais e internacionais, livros, capítulos, dissertações, teses e relatórios técnicos.
A elaboração do projeto do RI do IPEN foi iniciado em novembro de 2013, colocado em operação interna em julho de 2014 e disponibilizado na Internet em junho de 2015. Utiliza o software livre Dspace, desenvolvido pelo Massachusetts Institute of Technology (MIT). Para descrição dos metadados adota o padrão Dublin Core. É compatível com o Protocolo de Arquivos Abertos (OAI) permitindo interoperabilidade com repositórios de âmbito nacional e internacional.
O gerenciamento do Repositório está a cargo da Biblioteca do IPEN. Constam neste RI, até o presente momento 20.950 itens que tanto podem ser artigos de periódicos ou de eventos nacionais e internacionais, dissertações e teses, livros, capítulo de livros e relatórios técnicos. Para participar do RI-IPEN é necessário que pelo menos um dos autores tenha vínculo acadêmico ou funcional com o Instituto. Nesta primeira etapa de funcionamento do RI, a coleta das publicações é realizada periodicamente pela equipe da Biblioteca do IPEN, extraindo os dados das bases internacionais tais como a Web of Science, Scopus, INIS, SciElo além de verificar o Currículo Lattes. O RI-IPEN apresenta também um aspecto inovador no seu funcionamento. Por meio de metadados específicos ele está vinculado ao sistema de gerenciamento das atividades do Plano Diretor anual do IPEN (SIGEPI). Com o objetivo de fornecer dados numéricos para a elaboração dos indicadores da Produção Cientifica Institucional, disponibiliza uma tabela estatística registrando em tempo real a inserção de novos itens. Foi criado um metadado que contém um número único para cada integrante da comunidade científica do IPEN. Esse metadado se transformou em um filtro que ao ser acionado apresenta todos os trabalhos de um determinado autor independente das variáveis na forma de citação do seu nome.
